Plastids are a diverse group of double-membrane bound organelles found in most plants and algae. They may also be found in ferns, moss, parasitic worms and marine mollusks. Apart from photosynthesis, these organelles also assist in food storage and synthesis of compounds such as lipids, amino acids and carbohydrates. They also play a role in environment sensing, gravity sensing, and stomata regulation.
Plastids are both phylogenetically and physiologically diverse with varying color, size, and composition. For example, Leucoplasts that store nutrients are typically colorless, lacking any pigmentation. While, chromoplasts specialized for nutrient synthesis are equipped with specialized pigments which render the plant cell with its characteristic color.
Plastids can also be classified based on their structure and composition. Primary plastids are typically simple in structure, found in most autotrophs such as algae and plants. On the other hand, secondary plastids are more complex and can be rarely found in algae such as diatoms and protists such as dinoflagellates.
All life on earth is sustained by photosynthesis – a process by which chemical energy from the sun is transformed into nutrients that can be utilized by living organisms. Photosynthesis is also crucial for the conversion of carbon dioxide into oxygen which is essential for cellular respiration. In order to carry out this complex reaction, plants and other autotrophs are equipped with plastids.
DID YOU KNOW?
Scientists have recently discovered that some marine organisms such as slugs, flatworms and ciliates exhibit a unique phenomenon called kleptoplasty (Klepto meaning thief). Although these organisms do not contain their own plastids, they sequester plastid containing organisms such as algae into their body which are then partially digested, leaving behind their intact plastid. These plastids continue to perform photosynthesis, delivering nutrients to their host organism.

Plastid Function
Plastids play crucial roles in a variety of cellular functions.
- Plastids assist in the production of many essential plant components such as enzymes, lipids, Sulphur compounds and amino acids.
- In autotrophic eukaryotes, plastids are also the site of nutrient storage.
- Chloroplasts assist in photosynthesis, a process which sustains all life on earth.
- Plastids also synthesize their own chlorophyll chromatophores which facilitate light absorption.
- Plastids contain their own genome which facilitates self-replication, plant growth and differentiation.
- In algae, plastids assist in cellular respiration and nutrition.
- In parasitic eukaryotes which ingest plastids, these serve as additional carbon sources.
- Plastids engage in signaling with the nucleus which coordinates the expression of nuclear and plastid genes involved in plant growth and differentiation.
Structure of Plastids
Despite the dazzling diversity of plastids, they all share some common characteristics. They are typically small in size, approximately 5-10 microns in diameter and 3 microns in thickness. The shape of plastids can vary between discoid, spherical, dumbbell-shaped or lens-shaped, depending on the species of plant. All plastids are surrounded by a double layered envelope which enclose a cytosol like environment called the stroma.
The plastid envelope is composed of galactolipids such as galactolipid monogalactosyldiacylglycerol (MGDG) in complex with other proteins and lipids. However, the number of membranes can vary between plastids, depending upon the complexity of plastid function. For example, plastids involved in photosynthesis can contain up to 4 internal membrane layers.
In some cases, the internal membranes attach together to form a continuous structure called the peripheral reticulum. In addition to protecting the structural integrity of the plastid, these envelopes play a role in plastid communication, protein transport, signaling, gene expression, plant growth regulation and metabolism.
In some plant cells, plastids in the plant cytosol may be connected to each other via long thin protrusions arising from the envelope called stromules. Through these channels, components can be easily transported to and from the plastids. These stromules also facilitate communication and signaling between plastids. In complex eukaryotic cells, stromules can extend all the way through the cell up to the cell membrane.
The space enclosed within the plastid envelope is referred to as the stroma. Like the cytoplasm, the stroma is also filled by a colorless liquid embedded in matrix. While plastids specialized in storage such as leukoplasts may not contain any subcomponents to maximize space for storage, those involved in cellular processes may contain many specialized sub-organelles.
These may include photosynthetic machinery such as the thylakoids, protein manufacturing ribosomes, nucleoids, inclusion bodies, microtubules, starch globules, plastoglobules and stroma centers. Plastid genome can vary between 75-250 kbp, encoding for over 100 genes and is typically found attached to the inner plastid envelope/membrane. This DNA-membrane complex is referred to as a ‘nucleoid’.
The number of genome copies within a nucleoid can vary between plastid and cell types. For example, rapidly dividing cells contain more nucleoids than mature cells. Further during plastid division and differentiation, the size, shape and copy number of nucleoids can change. The plastid genome only partially encodes all the proteins and machinery required for plastid function, all other components are manufactured in the nucleus and transported to the plastid.
DID YOU KNOW?
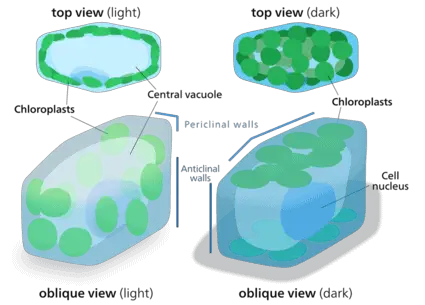
Plastids are dynamic structures that move within the plant cytoplasm. In order to facilitate their movement, plastids are connected to the cellular matrix embedded with networks of actin and myosin. The movement of plastids within a cell have been visualized in detail with chloroplasts. When a plant leaf is exposed to direct sunlight, the chloroplasts stack into columns towards the edge of the cell to avoid exposure. In contrast, in the dark, the plastids redistribute across the cytoplasm as sheets to maximize light absorption.
Types of Plastids
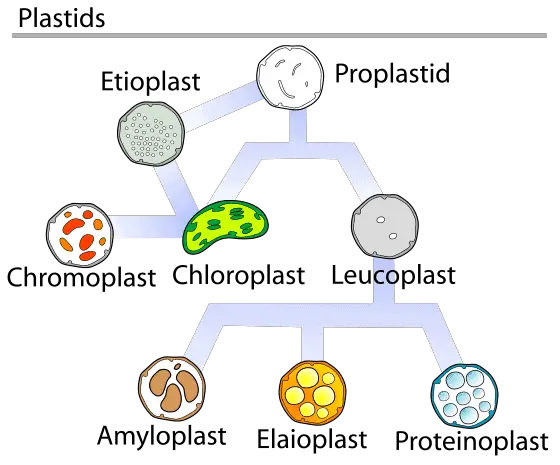
There are a diverse array of plastids found in plants and other eukaryotes, which have been specialized for distinct functions. Typically, all plastids develop from the progenitor proplastid. Further, one type of functional plastid may rapidly differentiate into another based on the cell’s requirement. A few common plastids are described below.
Eoplasts
Eoplasts are the primitive plastids which do not contain any complex structures. They are typically seen in young plant cells and subsequently differentiate into other subtypes based on requirement. Interestingly, recent evidence suggests that eoplasts cannot be formed by a young cell and are instead, inherited maternally. However, following inheritance, these eoplasts rapidly divide, providing the young leaf with plastids that can be differentiated to fulfil their functional needs.
Etioplasts
Etioplasts develop when leaves grow in dark environments for an extended period of time. Such leaves lose their characteristic green pigments and turn yellow. Etioplasts are made up of crystalline structures filled with nutrients which help the plant cells survive in the absence of photosynthesis. When the plant is re-exposed to light, the etioplasts differentiate back into chloroplasts.
Chromoplasts
Chromoplasts contain chromatophores (chroma meaning color) which render them with a characteristic color. These plastids may be found in colored leaves, flowers, fruits and some roots. Eukaryotic chromoplasts can range between the green colored chloroplasts, red colored rhodoplasts, brown colored phaeoplasts to yellow colored xanthoplasts. These colored plastids derive their color from distinct chromatophores or pigments which have been synthesised by the plastid themselves.
They serve multiple functions such as attracting insects for pollination, attracting animals to fruits for seed dispersion, or assisting in light absorption for photosynthesis. Chloroplasts are the most elaborately studied chromoplasts due to their role in photosynthesis and will therefore be explored in detail in this article.
Based upon structure, chromoplasts can be further classified as;
- Reticulo-tubular chromoplasts
- Globular chromoplasts
- Crystallised chromoplasts
- Tubular/fibrillar chromoplasts
- Membranous chromoplasts

Leucoplasts
Leucoplasts are colorless plastids specialized for storage of components. They are typically found in the non-green parts of the plant such as roots, seeds and tubers. Based on the component stored in these plastids, they can be further classified as
- Amyloplasts – These plastids store starch and help the root detect gravity by a phenomena called geotropism. They may also store enzymes and other components that assist the root in absorption of nutrients.
- Elaioplasts – These plastids are specialised for lipid storage. They scarcely contain any internal structures so as to maximize space for globular storage sacs. As a result elaioplasts are manipulated in industries and labs for the extraction of oil.
- Proteinoplasts – These plastids manufacture, store and modify protein components required by the plant cell. They are typically found in seeds, nuts and other protein rich regions.
- Tannosomes – These plastids are specialised for manufacturing, modifying and storing tannins. These are typically formed in the vascular regions of the plant when chloroplasts are modified by tannin accumulation. These plastids defend the plant from pathogens and UV radiation.
Plastid Division and Differentiation
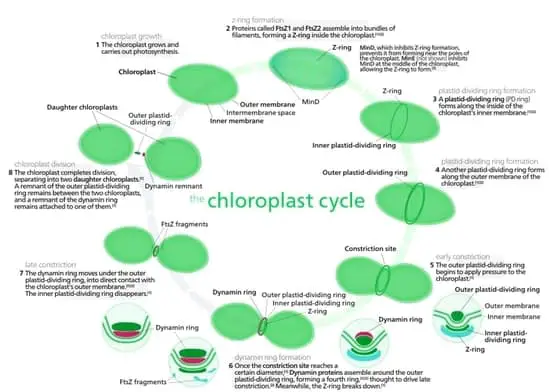
Advances in electron microscopy facilitated the stepwise elucidation of plastid division and differentiation. Plastids are typically inherited maternally, and subsequently undergo division. Plastid division begins with the formation of a construction through the middle of the plastid. This ring like constriction is also referred to as the Plastid dividing ring (PD).
Concurrently, the plastid stroma rapidly multiplies, resulting in the expansion of the plastid. Subsequently, this constriction narrows and forms the isthmus which connects the two daughter plastids. Finally, the separation of the daughter plastids is orchestrated by the movement of the dynamin protein network and the contractile ring in the plastid stroma.
However, it should be noted that scientists have also observed other distinct methods of plastid division several species of plants. For example, in peanut plants, chloroplast division occurs through the formation of membranous baffle across the center of the plastid. We do not yet know what factors influence the type of division chosen by plastids. Furthermore, the role of proteins in the stroma that facilitate plastid division is yet to be thoroughly explored.
Scientists do not fully understand what factors trigger plastid division. However, proplastids found in the meristematic cells of growing plants are in constant division. Similarly, during cell division, an equal number of plastids are distributed between the daughter cells. However, once these primitive proplastids differentiate into functional plastids, their ability to divide becomes limited. Finally, in cells exposed to harsh environments, plastids rarely divide and are typically converted to etioplasts until the environment becomes more favorable for plant growth.
DID YOU KNOW?
Arabidopsis thaliana also called the thale cress is a small flowering plant found in Europe, Asia and Africa. One mutant variety of this species contains a characteristic crumpled leaf. RNA analysis of these leaves revealed that the crumpled leaf variant contains mutations in genes which facilitate plastid division. As a result, in these plants, plastid division is suppressed leading to an unequal plastid distribution between the daughter cells. This results in growth abnormalities and the characteristic ‘crumpled’ leaf structure.

All functional plastids arise from primitive proplastids which are colorless, underdeveloped structures. These are typically found concentrated in dividing tissues such as the meristem. As tissues mature, proplastids differentiate and transform into functional plastids such as leucoplasts, chromoplasts or etioplasts. Environmental signals which facilitate plastid differentiation can include tissue maturation, fruit development, light, temperature, nutrition, etc.
Chloroplast differentiation is the most elaborately studied plastid differentiation pathways. When plant cells are exposed to light, proplastids or other plastid subtypes begin to manufacture and accumulate chlorophyll pigments. Subsequently, a complex internal membrane system develops along with other chloroplast substructures, resulting in the formation of a fully developed chloroplast.
Although plastids in research have been classified into distinct subtypes based on morphology and function, in a cell’s life cycle plastid interconversion is very dynamic. As a result, many scientist prefer to refer to plastids as a continuous spectrum of conversions. Some possible conversions between subtypes have been summarized in the figure.
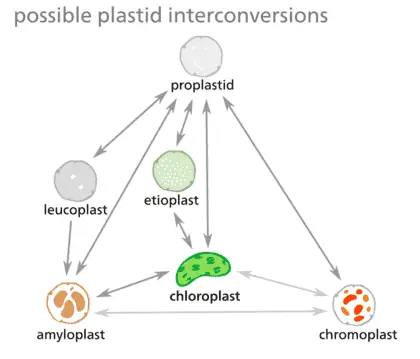
Chloroplasts and Photosynthesis
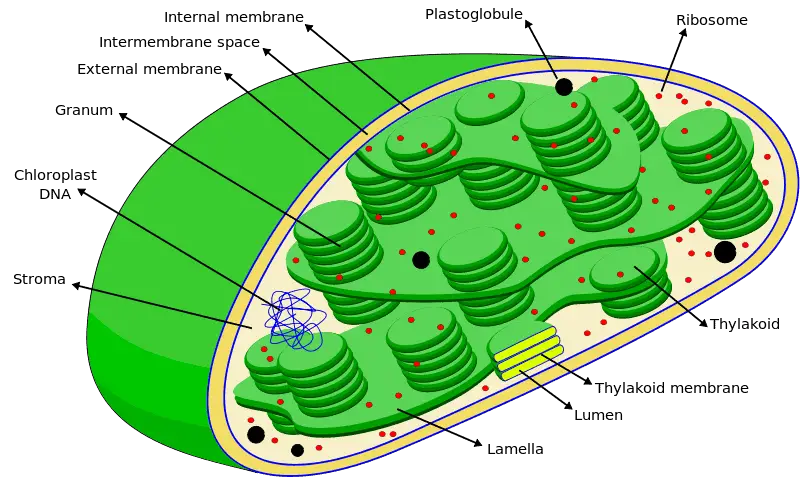
Chloroplasts (chloro meaning green) assist autotrophs in transforming chemical energy from the sun into glucose. They derive their name from green colored pigments called chlorophyll which give these organelles their characteristic color.
Chloroplasts are typically 1-2 um in thickness, and 4-6 um in diameter. Their shape can vary between spherical, oval or discoid based on the plant species and complexity. However, a typical plant cell contains up to 20-40 chloroplasts distributed evenly throughout the cytoplasm. During active photosynthesis or plant growth, this number may rise up to 200.
Chloroplasts are bound by two membranes, an outer envelope and an inner envelope which enclose an intermembrane space between them. Like the cell membrane, chloroplast membranes are made up of lipid proteins complexes.
Beyond the inner envelope lies the stroma filled with a cytosol like liquid and the grana. Grana are small cylindrical structures which are thought to have first evolved from an inner membrane folding inwards. A typical chloroplast may contain up to 100 granas. Each grana, in turn, consists of disc shaped thylakoids stacked over one another into a tight column.
The thylakoids are hollow structures which enclose an aqueous compartment called the lumen which is filled with chlorophyll. Therefore, these structures are the primary light harvesting system in the chloroplast. Grana are connected to one another through membrane like channels called stroma lamellae. In addition to grana, stroma may also contain enzymes, starch, plastid genome and ribosomes.
Taken together, the chloroplast can be divided into three primary regions namely; The intermembrane space enclosed between the double membrane, the stroma enclosed within the internal envelope and the Thylakoid lumen containing chlorophyll.
In addition to colored pigments, the thylakoid lumen also houses the protein complexes that carry out light dependent photosynthesis. These include the photosystem 1, photosystem 2 and ATP synthase. When light strikes the chloroplast present on plant surfaces, the colored pigments in the thylakoid assist in light absorption. Some common pigments or chromatophores found in chloroplasts include chlorophyll, carotenoids and phycobilins which absorb light at distinct wavelengths.
| Chlorophyll A | 400 – 450 nm, 650 -700 nm |
| Chlorophyll B | 450 – 500 nm, 600 – 650 nm |
| Carotenoids | 460 – 550 nm |
| Xanthophyll | 400 – 530 nm |
DID YOU KNOW?
Green light exists at wavelength 520- 560 nm. As we can see from the table of pigments found in chloroplasts, none of them absorb light in this wavelength range. This is why parts of the plant containing chloroplasts appear green in color.

When light is absorbed by the chromatophores, the electrons in the pigment leave the molecule and enter a series of concurrent reactions which are collectively referred to as the electron transport chain (ETC). The Photosystems protein complexes act has electron donors while ADP acts as an electron acceptor.
ATP synthetase present in the thylakoid lumen also assists in the phosphorylation of ADP, resulting in the formation of the high energy storage compound Adenosine triphosphate (ATP). In addition to ATP, the electron transfer chain also assists in the phosphorylation of NADH resulting in the formation of nicotinamide adenine dinucleotide phosphate (NADPH).
Taken together, all these pathways constitute the light dependent portion (light reactions) of photosynthesis. Subsequently, ATP and NADPH are used to power the light independent reactions (dark reactions) of photosynthesis by which carbon dioxide and water are integrated into organic compounds, thus resulting in the formation of glucose. While the light dependent portion occurs in the thylakoid lumen, the dark reactions take place in the chloroplast stroma.
The equation for the light and dark reactions of photosynthesis can be represented in the following way;
(Carbon Dioxide) 6CO2 + (Water) 6 H2O → (Glucose) C6H12O6 + (Oxygen) 6O2
The Discovery of Plastids
The study of plastids has played a key role in the elucidation of plant physiology and biochemistry. These structures were first identified and described by Ernst Haeckel in 1866.

In 1880, A.F.W Schimper identified the role of plastids in photosynthesis. He coined the term – plastid (plastikas meaning moulded or formed) based on their plastic nature to consistently change in morphology.
Subsequently, in 1905, the Russian biologist K.S Mereschkowsky first identified the plastids which give leaves their characteristic green color – chloroplasts. He identified that these plastids contain colored pigments called chromatophores which assisted in performing photosynthesis. He also theorised that eukaryotes obtained chloroplasts by forming symbiotic relationships with bacteria. Yet, for over a century, the importance of these organelles remained unknown.
In the 1960s, the development of the electron microscope enabled the elucidation of the plastid’s complex structure and composition. Subsequently, several important discoveries were made including pigment morphology, storage and identification of other plastid substructures. In 1977, J.V Possingham and group observed young chloroplasts to describe the process of plastid division.
Towards the end of the 1970s, plastids began to be classified by the complexity and morphology of their substructures. For example, plastids in pumpkin leaves were found to contain plastoglobules filled with pigments, thus earning them the name globular chromoplasts. Through the study of plant physiology, members of the diverse plastid family were found to be crucial roles in many essential life processes apart from photosynthesis. Consequently, plastid biology continues to be a hot topic for research among plant biologists to this day.
How Did Plastids Evolve?
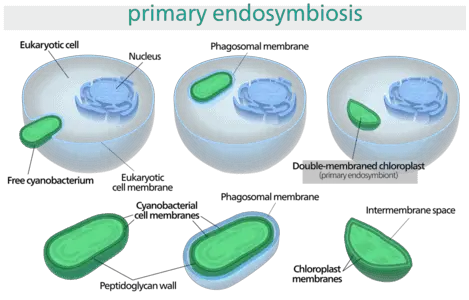
The study of plastids has provided incredible insight into the origin of photosynthesis and thus, the evolution of our food chain. Plastids are thought to have originated in eukaryotes over 1.5 billion years ago through endosymbiotic relationship with cyanobacteria. This theory was largely controversial when it was first proposed by K.S Mereschkowsky in 1909.
He theorized that since plastids such as chloroplasts are capable of dividing independently, possess an independent genome, and function as independent organisms, they could be regarded as endosymbionts. He argued that eukaryotic mitochondria and plastids arose by ‘symbiogenesis’, a process by which an eukaryotic cell engulfed a cyanobacterium and retained it within its membrane. Subsequently, a double membrane formed around the plastid following its integration with other cellular organelles.
Since the 18th century, advancements in molecular biology, biochemistry and plant physiology have contributed further proof to support the endosymbiosis theory. For example, genetic analysis has revealed that plastids possess a circular double stranded DNA molecule similar to prokaryotic cells. During evolution, many non-functional prokaryotic genes have been deleted from the plastid genome, leaving behind genes that exclusively code for plastid function.
However, to this day, some remnants of the prokaryotic genome can be found in plastids. The primary drive for symbiogenesis is purported to be photosynthesis. However, since then plastids have evolved to become an essential component of plant cell function, playing a role in lipid biosynthesis, amino acid metabolism, fruit ripening, endosperm development and root gravitropism.
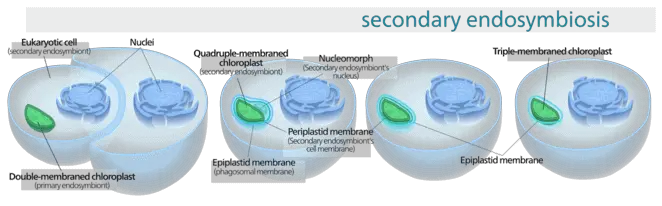
However, this theory only explains the origin of primary plastids. Secondary plastids containing a more complex structure are thought to have arisen more recently, about 60 million years ago, through a secondary endosymbiotic event with cyanobacteria wherein a plastid containing eukaryotic cell engulfed an additional plastid. Thus secondary plastids may possess up to two- three additional membranes.
Differences Between Plastids and Mitochondria
Although mitochondria and plastids share a common evolutionary link through endosymbiosis, there are some distinct differences between the two organelles.
| MITOCHONDRIA | PLASTIDS |
| Exists in all eukaryotes | Present only in autotrophs |
| Produces ATP by cellular respiration | Produces glucose by photosynthesis |
| Smaller than plastids | Comparatively large |
| Does not contain chromatophores | Contains chromatophores |
References
- Archibald, J. M. The puzzle of plastid evolution. Current Biology 19, R81–R88 (2009) doi:10.1016/j.cub.2008.11.067.
- Delwiche, C. F. Tracing the thread of plastid diversity through the tapestry of life. American Naturalist 154, S164–S177 (1999) doi:10.1086/303291.
- Griffin, K. L., & Seemann, J. R. (1996). Plants, CO2 and photosynthesis in the 21st century. Chemistry & Biology, 3(4), 245–254. https://doi.org/10.1016/S1074-5521(96)90104-0
- Jensen, P. E., & Leister, D. (2014). Chloroplast evolution, structure and functions. F1000Prime Reports, 6. https://doi.org/10.12703/P6-40
- Johnson, M. P. (2016). Photosynthesis. Essays in Biochemistry, 60(3), 255–273. https://doi.org/10.1042/EBC20160016
- Marin, B., Nowack, E. C. M. & Melkonian, M. A plastid in the making: Evidence for a second primary endosymbiosis. Protist 156, 425–432 (2005). doi:10.1016/j.protis.2005.09.001
- Moreira, D., Le Guyader, H. & Philippe, H. The origin of red algae and the evolution of chloroplasts. Nature 405, 69–72 (2000) doi:10.1038/35011054.
- Ponce-Toledo, R. I., Deschamps, P., López-García, P., Zivanovic, Y., Benzerara, K., & Moreira, D. (2017). An early-branching freshwater cyanobacterium at the origin of plastids. Current Biology : CB, 27(3), 386. https://doi.org/10.1016/j.cub.2016.11.056
- Reyes-Prieto, A., Weber, A. P. & Bhattacharya, D. The origin and establishment of the plastid in algae and plants. Annual Review of Genetics 41, 147–168 (2007) doi:10.1146/annurev.genet.41.110306.130134.
- Sadali, N. M., Sowden, R. G., Ling, Q., & Jarvis, R. P. (2019). Differentiation of chromoplasts and other plastids in plants. Plant Cell Reports, 38(7), 803. https://doi.org/10.1007/s00299-019-02420-2
- Steenkiste, N.W.L.V., Stephenson, I., Herranz, M., Husnik, F., Keeling, P.J., Leander, B.S., 2019. A new case of kleptoplasty in animals: Marine flatworms steal functional plastids from diatoms. Science Advances 5, eaaw4337. https://doi.org/10.1126/sciadv.aaw4337
- Wise, R. R., & Hoober, J. K. (2006). The Structure and Function of Plastids. Springer.
- Y, C., T, A., Mt, F., S, Y., Y, M., & Y, Y. (2009, May). Plant Cells Without Detectable Plastids Are Generated in the Crumpled Leaf Mutant of Arabidopsis Thaliana. Plant & Cell Physiology; Plant Cell Physiol. https://doi.org/10.1093/pcp/pcp047
- Zechmann, B. (2019). Ultrastructure of plastids serves as reliable abiotic and biotic stress marker. PLOS ONE, 14(4), e0214811. https://doi.org/10.1371/journal.pone.0214811