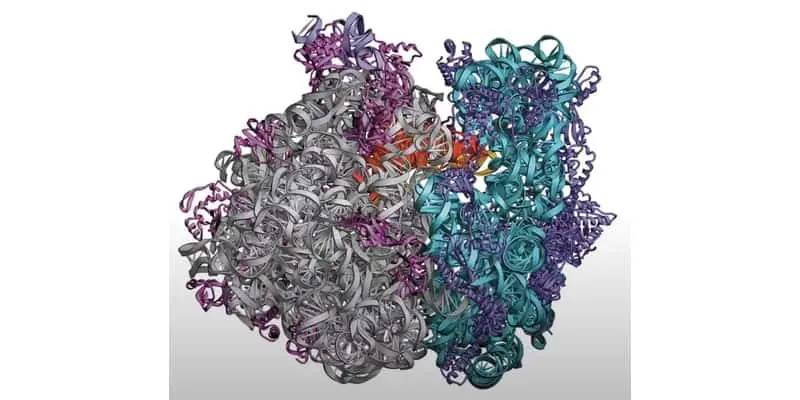
Ribosomes are cellular organelles made up of granular ribonucleic acid and ribosomal protein complexes that facilitate the synthesis of proteins from messenger RNAs. In a way, these organelles are one of life’s most ancient “factories” on the planet.
Communication among living organisms takes many forms. While most animals communicate through sound, others correspond using touch, smell or the release of chemicals. Interestingly, this diversity does not translate to the building blocks that make up life. Cells talk to one another by transferring information through distinct molecules.
The DNA in most organisms and RNA in viruses is an encyclopedia, containing all the information required to sustain life. However, these molecules are of substantial size and cannot be moved freely across the cell. For this purpose, the information stored in the genetic material is translated into smaller macromolecules that can be transported across the body.
Proteins are versatile molecules made up of amino acids stacked into long chains called polypeptides. These macromolecules are key players in all cellular and molecular processes taking place in the body. They are the building blocks of many central structures ranging from the bones that build the cytoskeleton to the complexes that form the cell wall.
Some specialized proteins called enzymes help speed up chemical reactions, while immune complexes made of proteins protect the organism from invaders. Further, when cells need to communicate with one another, they secrete proteins which travel between cells with the message. The receptors or ‘antennae’ that receive and recognize these signals are also constructed with proteins.
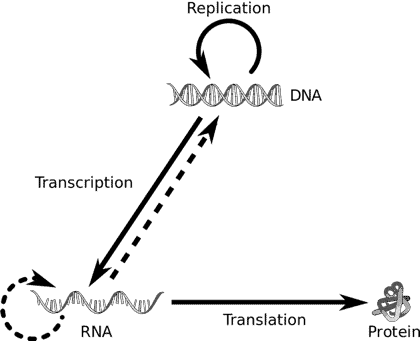
Most life on earth follows this identical pathway of information transfer wherein DNA (deoxyribonucleic acids) is transcribed to form messenger RNAs (Ribonucleic acids) which are then translated into proteins. This directional flow of information is referred to as the central dogma of molecular biology.
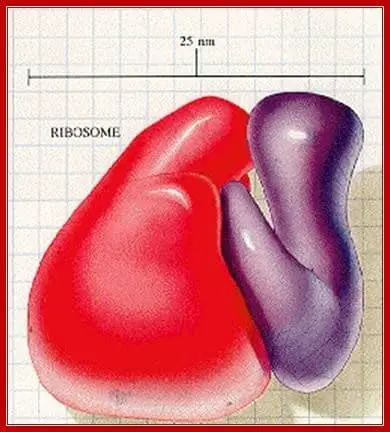
Unlike other cell organelles, ribosomes are found in both prokaryotic and eukaryotic cells alike. However, prokaryotic ribosomes are significantly smaller than their eukaryotic counterparts.
The table below shows the differences between prokaryotic and eukaryotic ribosomes.
| PROKARYOTES | EUKARYOTES |
|---|---|
| A single cell can contain up to 15,000 ribosomes. | A single cell contains over 10 million ribosomes. |
| Size ranging between 45 – 70S | Size up to 80S |
| Composition – 65% RNA and 35% protein | Composition – 50% RNA and 50% protein |
| Found scattered across the cytoplasm | Can be present in the cytoplasm or attached to the surfaces of organelles. |
DID YOU KNOW? - Some scientists do not consider ribosomes to be cell organelles because they lack a typical phospholipid membrane. Ribosomes, unlike other cell-organelles are simply particulate and are therefore referred to as non-membranous organelles.
Where are Ribosomes Located?
In prokaryotic cells, which do not contain well-defined organelles, ribosomes are found scattered across the cytoplasm. However, in eukaryotic cells, these can exist in two different forms. Free ribosomes are not restricted to a specific location in the cytosol. They can generally move across the cell surface, as required. Due to their location, these ribosomes are used exclusively in the synthesis of proteins that are utilized within the cell.
Further, the cytoplasm maintains a highly reducing environment which promotes an electrochemical gradient ideal for electron transfer. Therefore, these free ribosomes only synthesize proteins that can survive in this highly reductive environment. In contrast, organelles that require protein synthesis may also contain ribosomes attached to their cell surface.
These ribosomes, called membrane bound ribosomes can be found on the nucleus, mitochondria, endoplasmic reticulum (ER) and the chloroplast. The ER, in particular, is a critical player in protein synthesis. In most eukaryotic cells, the ER is bound to several thousand ribosomes appearing as a granular complex commonly referred to as the ‘rough’ endoplasmic reticulum. It is called so because it appears bumpy under the microscope.
DID YOU KNOW? - The oocytes of lizards have a very unique composition of ribosomes. In these creatures the ribosomes are organized into a crystalline sheet where they remain until the cells need them to synthesize proteins.

Structure of Ribosomes
Ribosomes are granular complexes made up of ribonucleic acids and ribosomal proteins. The RNAs that make up ribosomes are referred to as ribosomal RNAs (rRNA). These are typically transcribed in the nucleus and transported to the cytoplasm during ribosome formation.
A typical ribosome can contain over 3 ribosomal RNAs coiled around each other, in complex with up to 80 ribosomal proteins. However, the exact number can vary widely depending on the species. Below is a brief summary of the composition of the most widely studied prokaryotic Escherichia coli ribosome and eukaryotic rat ribosome.
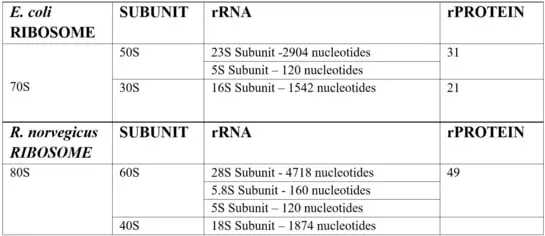
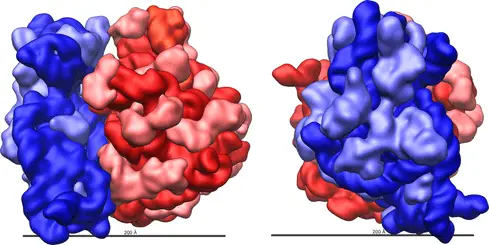
Ribosome complexes, irrespective of species, are made up of two subunits of varying sizes. The smaller unit connects the larger subunit to the messenger RNA (mRNA). While the larger subunit binds specialized structures called transfer RNA (tRNA). Transfer RNAs are small molecules composed of 70-90 nucleotides folded in a cross-shaped structure.
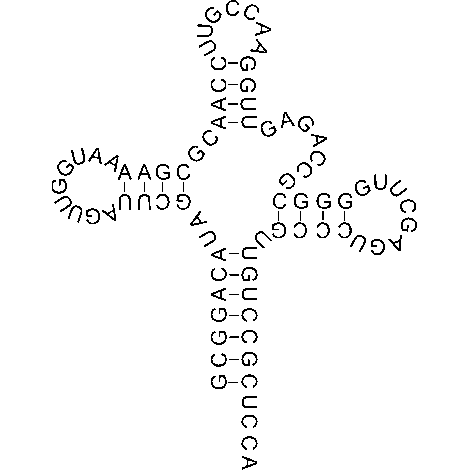
In addition to serving as the physical link between ribosomes and the newly formed polypeptide, these structures recruit amino acids based on the information contained in the mRNA sequence. The complex containing the ribosome and other components required in protein translation are collectively referred to as the translational apparatus.
There are several differences between ribosomes in prokaryotes and eukaryotes. A single Eukaryotic cell can require up to 10 billion proteins, and is thus equipped with over 10 million ribosomes. In contrast, a typical prokaryotic cell such as Escherichia Coli only contains up to 15,000 ribosomes. Ribosomes also differ in size between species. Interestingly, the size of ribosomes is not measured by mass. Instead, scientists denote ribosome size by the Svedberg unit (S), which measures the sedimentation rate or the rate at which a molecule moves through a fluid medium.
A prokaryotic ribosome typically weighs up to 70S while an Eukaryotic ribosome can weigh about 80S. Prokaryote 70S ribosomes are in turn made up of one larger 50S subunit and a smaller 30S subunit. In contrast, eukaryotic ribosomes generally contain a combination of 60S and 40S subunits.
You might notice that unlike the mass of a molecule, the Svedberg units do not add up! Finally, the size of a ribosome can also vary depending on the stage of cell growth. For example, a cell in division will require more proteins and thus contains larger ribosomes. Prokaryotic ribosomes, which evolved earlier, also contain a greater proportion of RNA compared to eukaryotic ribosomes. While prokaryotic ribosomes contain up to 60% ribosomal RNA, eukaryotic ribosomes are composed of equal parts protein and RNA.
DID YOU KNOW? - The differences between prokaryotic and eukaryotic ribosomes have been exploited to design drugs such as antibiotics. These drugs specifically target bacterial ribosomes, thereby reducing the damage caused to host cells.
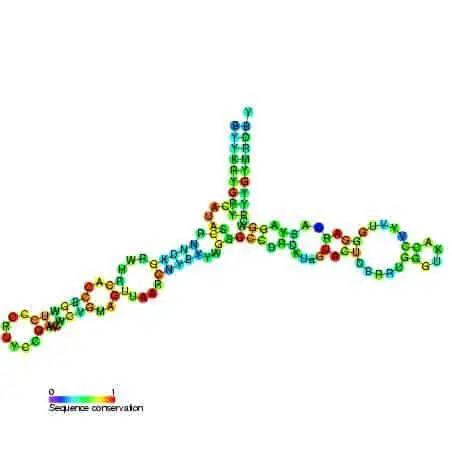
Yet, despite all these differences, most functionally active sites on the ribosome such as mRNA and tRNA binding sites are conserved across many species. Advances in X-ray crystallography in the past two decades have enabled the elucidation of ribosome composition and structure. The shape of a ribosome subunit is determined by the structure of its composite rRNA.
Biochemical and genetic research has further confirmed that all the functionally important sites of a ribosome are present in its rRNAs. In contrast, ribosomal proteins primarily serve to stabilize the structure of the ribosomes. These proteins found attached to interhelical sites, stabilize the attractive and repulsive forces between the ribosomal complex. These proteins cluster around the rRNA and the periphery of the subunits, facing the solvent side of the cytoplasm.
Despite being present only on the surface of the complex, ribosomal proteins contain large polypeptide chains that penetrate deep into the core of the ribosomal complex. However, most active sites on the rRNA structure stay exposed for target binding, without any proteins in its vicinity. The larger subunits are generally more rigidly packed and consequently contain more ribosomal proteins that function as catalyzers. As a result, the larger subunit is often referred to as ribozyme.
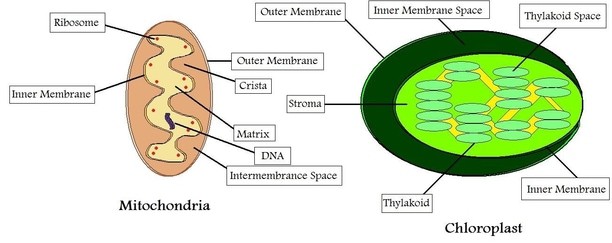
DID YOU KNOW? - The ribosomes bound to eukaryotic mitochondria (mitoribosomes) and chloroplasts (plastoribosomes) resemble the ribosomes found in prokaryotic cells in both size and composition. As a result, wouldn’t antibiotics targeted at bacterial ribosomes damage host cells via their mitochondria? You’ll notice from the figure that mitoribosomes are enclosed within a double layered membrane which is impenetrable to antibiotics. In this way, the host cells remain unaffected.
How are Ribosomes Formed?
The proteins and nucleic acids that compose ribosomes are synthesised in a small compartment inside the nucleus called the nucleolus. Following transcription of ribosomal RNA components required in ribosome synthesis, the RNAs are transferred to the nucleolus. These pre-RNAs need to be edited in order to yield mature rRNA. However, there are distinct differences in post-transcriptional modifications between prokaryotes and eukaryotes. In prokaryotes such as E.coli, enzymes catalyse the formation of covalent bonds. In contrast, in eukaryotes post-transcriptional modifications include formation of pseudouridine and methylation of sugar residues.
The sites of these modifications are selected and marked by small protein complexes called small nucleolar ribonucleoprotein (snoRNP). A single human cell can contain over 100 different types of snoRNPs, each with a unique role in ribosome formation. Subsequently, during maturation, pre-ribosomal RNA bound with ribonucleoproteins are transported to the cytoplasm via the nuclear pore complex (NPC).
How do cells know which molecules should be transported out of the nucleus? Just like in post-offices where mail is assigned specific post codes depending on where they need to go, the mRNAs are embedded with specific sequences or barcodes which decide where these nucleotides will be transported. However, transport of these molecules does not occur freely.
These are energy consuming processes, catalyzed by GTP/ATP and specialized transporter complexes in the NPC. This ensures that transport through the nucleus is highly specific and does not occur without a purpose. The protein complexes that facilitate transport are highly sophisticated miniature machines and scientists are continuing to elucidate their mechanics to this day.
The final maturation of the pre-ribosomal particles takes place in the cytoplasm, where these particles are folded to stabilize their final structure. However, ribosomes only have a temporary existence in the cell. When the required proteins have been synthesized, ribosomes are typically broken down. The raw materials may then be recycled or simply degraded and excreted.
DID YOU KNOW? - Ribosomopathies are diseases which result from mutations that interfere with the synthesis and function of ribosomes. Mutations in the proteins that compose ribosomes have been linked to bone marrow diseases and anemia. Interestingly, some ribosomal mutations have also been linked with an increased risk of developing cancer in later life.
Protein Synthesis in Ribosomes
The primary function of ribosomes is to synthesize proteins. They are highly efficient machines that can link over 200 amino acids in a single minute. In some instances, several ribosomes can decode a single mRNA sequence at the same time. Such a complex is referred to as a polysome.
The formation of proteins can be split into two steps:
- The decoding of the genetic code
- The formation of peptide bonds between amino acids
Let us look at the first step; decoding. The cells of life exhibit an extraordinary variety of proteins and other molecules.
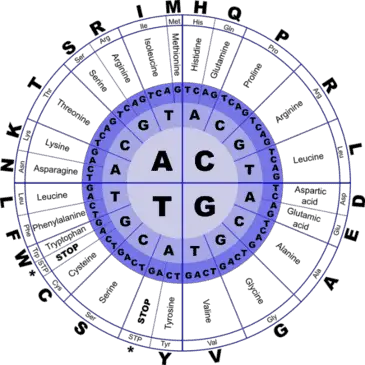
How is it that all this variety can be designed by a single language containing just 4 alphabets? Life on earth begins with the genetic code containing four types of nucleotides arranged in distinct patterns– Adenine (A), Guanine (G), Thymine (T) and Cysteine (C). However, when DNA is transcribed to form messenger RNA, the thymine molecule is replaced with uracil, a nucleotide which lacks a methyl group.
Uracil is energetically less costly to produce and therefore more cost effective for the cell. Once mRNA is transcribed, several post-transcriptional modifications take place before these nucleotides can be transferred to the cytoplasm for the formation of proteins.
Every three nucleotides in the mRNA – termed a ‘triplet codon’ codes for a specific amino acid. Take a look at the figure, reading from the center of the circle to the outside, we see that several triplet codons can code for the same amino acid. For example, the sequences AGT, AGC, TCA, TCG, TCT, TCC all code for the same amino acid- serine.
Now take a look at the parts of the outer circle titled ‘stop’. These are the codons which signal the ribosome to stop protein production. This way the cell ensures that ribosomes do not translate the signal sequences present at the end of mRNAs which are simply there to denote where the mRNA should be transported.
Using this complex coding process, a language that begins with four letters (nucleotides) is transcribed into 64 different combinations (triplet codons) which in turn translate into 20 different amino acids! An average human makes over 1020 proteins per hour. Isn’t it amazing that these tiny machines perform such complex functions with such a low rate of error?
The decoding of the messenger RNA is performed by the smaller subunits of both eukaryotic and prokaryotic ribosomes, while the larger subunits recruit amino acids and catalyse the formation of peptide bonds. Three main sites on the ribosomal structure are central to protein production and these are generally conserved across species.
- Site A (aminoacyl) – Site of mRNA binding and ‘charged’ tRNA recruitment.
- Site P (peptidyl) – Site of peptide bond formation and elongation of the polypeptide.
- Site E (exit to cytoplasm) – Site of exit of uncharged tRNA to the cytoplasm.
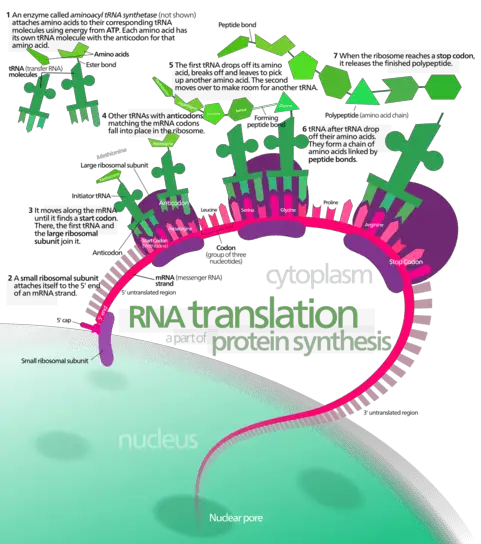
Both Site A and P constitute large portions of both the larger and smaller subunit while site E is present on the larger subunit of the ribosome. Protein production can be divided into three main stages namely; initiation, elongation and termination.
In the initiation stage, the small subunit of the ribosome binds the mRNA through a cleft present on its surface, while the initiator tRNA is recruited through another cleft. This movement of nucleotides through the subunit, recruits the larger subunit and locks all members of the translational complex into place.
At this stage, protein formation is ready to begin. The first codon in the mRNA recognized by the ribosome is merely a ‘start’ codon. These codons simply indicate the position in the mRNA where translation should start. Subsequently, a charged tRNA (see figure) with a specific amino acid corresponding to the identified codon in the mRNA is recruited to site A. The selection of tRNA that can bind to site A is highly specific. However, in some cases errors do occur and the wrong amino acid is added to the protein.
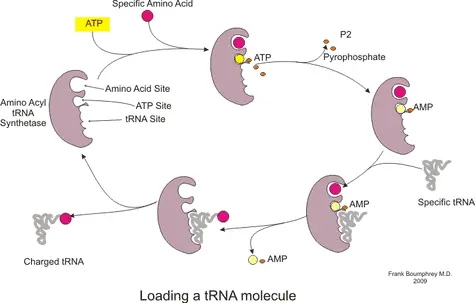
In this manner, during the elongation stage, the ribosome traverses the length of the mRNA reading every 3 nucleotides (codon) and recruiting the corresponding charged tRNA. Consecutively, the P site binds a tRNA charged with peptidyl transferase, an enzyme which catalyses the formation of a peptide bond between the amino acid. As the mRNA moves to the next codon, the now uncharged tRNA is moved to the E site on the ribosome from where it is released to the cytoplasm. This cycle repeats itself , the number of repetitions depending on the total number of codons in the mRNA. The polypeptide chain continues to grow until the mRNA reaches the ‘stop’ codon.
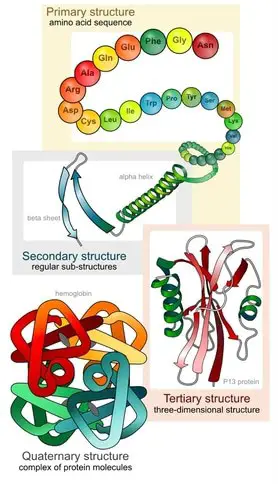
In the final termination stage, a set of proteins which facilitate the release of the polypeptide chain are recruited to the A site. The newly formed polypeptide chain is released through a cleft on the larger subunit, resulting in the disengagement of the two ribosome subunits. Subsequently, all the ribosomal components may be degraded or recycled. However, the newly formed polypeptide chain only represents the primary structure of the protein.
The chain is folded into several levels of complex structures with the assistance of accessory molecules. The folding of the protein chain can occur through two distinct processes. In the post-translational modification pathway, proteins are folded in the ER or cytoplasm after they have been released by the ribosome. In contrast, in the co-translational folding pathway, the polypeptide chain is folded with the assistance of ribosomes during the translational process. Electron microscopy helped reveal that the rotation of one subunit in relation to another drives the folding of the polypeptide chain into specific shapes. The movement of the subunits in turn are driven by ATP hydrolysis.
Evolution of Ribosomes
According to scientists, before the evolution of ribosomes, life was nucleotide based. All the key molecules involved in life’s cellular and molecular mechanisms were ribonucleotides (RNA) or deoxyribonucleotides (DNA). At this time, primitive ribosomes are thought to have originated as self-replicating RNA complexes. When amino acids first developed, they are thought to have formed bonds with the primitive ribosome as enzymes, thus catalyzing their reactions. This is thought to have been the evolutionary drive that promoted the transition of
ribosomes to a protein-RNA complex capable of synthesising new proteins. With this development, life switched from a nucleic-acid based world to a protein-based world.
Prokaryotic ribosomes evolved first, slowly developing the ability to recognize, bind and fold RNA molecules. Subsequently, these organelles gained the ability to associate with proteins to form the modern ribosome capable of synthesizing polypeptides. In eukaryotes, a drive for accumulation of more functional proteins is thought to have led to a tentacle like expansion resulting in the formation of the much larger eukaryotic ribosome.
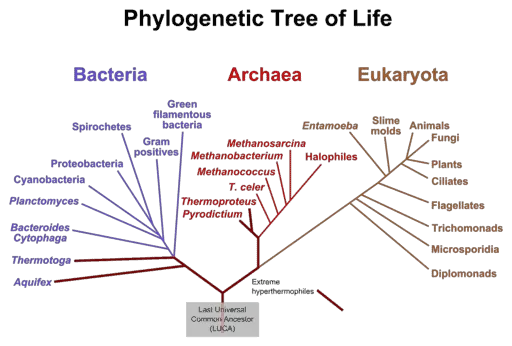
Due to the highly conserved structure of ribosomes which have been passed down from ancient archaic bacteria to the most complex eukaryotes on the planet, scientists have often looked toward studying these organelles to understand the phylogenetic relationships between organisms. The genetic sequence of ribosomes retains information about primordial cells from over 4 billion years ago!
To this day, the ribosomes of Archaea, Bacteria and Eukarya share a significant similarity in their functional elements. When their sequences are aligned against each other, three common blocks or domains can be found; one block which is conserved between bacteria, one which is present in both Archaea and Eukarya and one which evolved exclusively in Eukarya.
When Were Ribosomes Discovered?
Ribosomes were first described by an American Cell biologist, George Emil Palade in 1953. When he observed eukaryotic cells under an electron microscope, he noticed small granules associated with the endoplasmic reticulum.
Subsequently, the scientist Richard Roberts coined the term ‘ribosomes’ to refer to these dense granules. Ribosomes are named after their primary component - ribonucleic acids, and the Latin word ‘soma’ meaning body. Subsequently, George Palade along with Albert Claude and Christian de Duve was awarded the Nobel prize in Physiology or medicine in 1974 for their discovery of the role of ribosomes in protein production.
Tissieres and Watson in 1958 first isolated ribosomes from E.coli cells thus enabling the elucidation of its structure. They reported that these granular structures accounted for over 1/4th of the cell’s volume and 3/4th of its RNA composition. At this time, only the primary structure of the granular ribosome was known.
However, advancements in cryo-electron microscopy and X-ray crystallography in the early 2000s enabled the resolution of the ribosomal structure to the order of a few angstroms. For their discovery of both prokaryotic and eukaryotic ribosomal structure and mechanism, Venkatraman Ramakrishnan, Thomas Steitz and Ada Yonath were awarded the Nobel prize in Chemistry in 2009.
References
- Ban N, Nissen P, Hansen J, Moore PB, Steitz TA 2000. The complete atomic structure of the large ribosomal subunit at 2.4 A resolution. Science 289: 905–920
- Bashan A, Yonath A 2008. Correlating ribosome function with high resolution structures. Trends Microbiol 16: 326–335
- Bhuta A, Quiggle K, Ott T, Ringer D, Chladek S 1981. Stereochemical control of ribosomal peptidyltransferase reaction. Role of amino acid side chain orientation of acceptor substrate. Biochemistry 20: 8–15
- Caetano-Anolles G 2002. Tracing the evolution of RNA structure in ribosomes. Nucleic Acids Res 30: 2575–2587
- Davidovich C, Belousoff M, Bashan A, Yonath A 2009. The evolving ribosome: From non-coded peptide bond formation to sophisticated translation machinery. Res Microbiol. Jul 18
- Fox G. E. (2010). Origin and evolution of the ribosome. Cold Spring Harbor perspectives in biology, 2(9), a003483.
- Hsiao, C., Mohan, S., Kalahar, B. K., & Williams, L. D. (2009). Peeling the onion: Ribosomes are ancient molecular fossils. Molecular Biology and Evolution, 26(11), 2415–2425.
- Klein DJ, Moore PB, Steitz TA 2004. The roles of ribosomal proteins in the structure assembly, and evolution of the large ribosomal subunit. J Mol Biol 340: 141–177
- Lafontaine, D. L. J., & Tollervey, D. (2001). The function and synthesis of ribosomes. Nature Reviews Molecular Cell Biology, 2(7), 514–520.
- Narla, A., & Ebert, B. L. (2010). Ribosomopathies: human disorders of ribosome dysfunction. Blood, 115(16), 3196–3205.
- Nissen P, Hansen J, Ban H, Moore PB, Steitz TA 2000. The structural basis of ribosome activity in peptide bond synthesis. Science 289: 920–930
- Ramakrishnan V, White SW 1998. Ribosomal protein structures: insights into the architecture, machinery and evolution of the ribosome. Trends Biochem Sci 23: 208–212
- Schuwirth BS, Borovinskaya MA, Hau CW, Zhang W, Vila-Sanjurjo A, Holton JM, Cate JH 2005. Structure of the bacterial ribosome at 3.5 A resolution. Science 310: 827–834
- Spirin AS 2002. Ribosome as a molecular machine. FEBS Lett 514: 2–10
- Zhang B, Cech TR 1997. Peptide bond formation by in vitro selected ribozymes. Nature 390: 96–100
- Zimmerman E, Yonath A 2009. Biological implications of the ribosomes's stunning stereochemistry. Chembiochem 10: 63–72