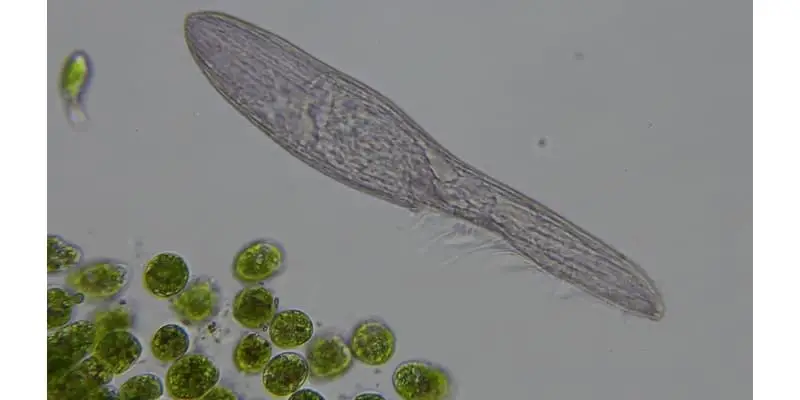
Among the myriad of unicellular protozoans that abound in both fresh and saltwater environments, Blepharisma may be the least well-known of the group.
Blepharisma is a commonly found, unicellular protozoan that is easily identified by its distinct pink coloration due to the presence of a pigment called blepharismin. Blepharisma are found in pond water most anywhere, though they have also been found in brackish water and sea water environments. Blepharisma range in size from 50 to 300 micrometers in length.
Blepharisma are normally a benign and non-threatening organism, finding its place in the world as the subject of much scholastic study in public science classrooms, as well as prolific academic research at universities all over the world. However, they can be highly toxic and fatal to humans in some cases which we will review later in this post. Blepharisma are easily cultured in the laboratory and are available from numerous scientific supply houses since they are such a common studied organism.
Blepharisma are classified in the following way:
- Kingdom: Protozoan
- Phylum: Ciliophora
- Class: Ciliatea
- Subclass: Spirotricha
- Order: Herterotrichida
Blepharisma Structure
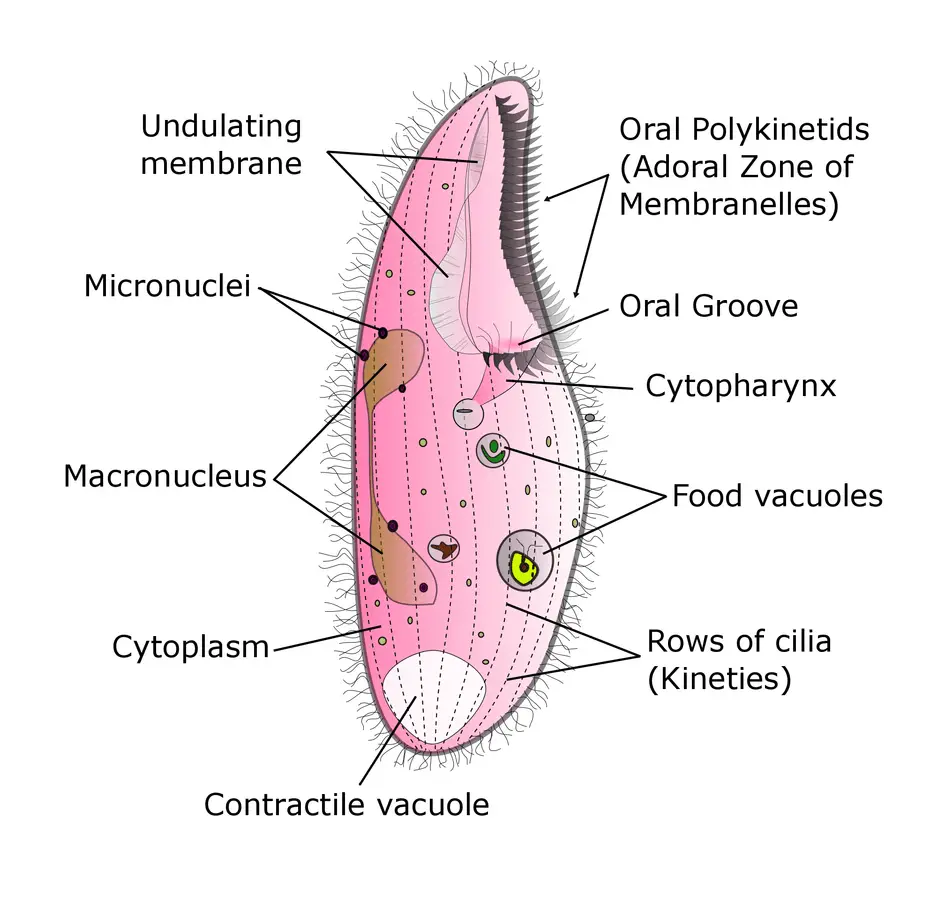
Blepharisma itself can be as small as 50 micrometers in length or as large as 1000 micrometers however, the normal range of size falls between 75 and 300 micrometers. They are ovoid in shape, overall, tapering off on one end, though the specific shape varies from species to species. Blepharisma is a genus of unicellular, ciliated protozoa using longitudinal rows of cilia along its body for locomotion as well as the intake of food.
All members of the genus contain an elongated series of various membranelles or organelles along the left-hand side of the organism along what has been termed the oral groove. Along the right-hand side, you will find the so-called “undulating membrane”. This feature is a flap made up of longer cilia fused together into a single sheet that leads toward the posterior of the organism.
For a short video to see how Blepharisma move about and to get an active view of the cilia at work as well as seeing some of the internal organelles of the organism, see the following YouTube clip ( the pink color normally seen in the organisms is absent here due to the way in which the photography was designed) :
The most unique characteristic of Blepharisma is its red or pinkish hue. The coloration comes from the possession of the pigment, blepharismin. This pigment can be found as granules just beneath the plasma membrane of the organism. Generally, Blepharisma are photo-phobic seeking out darker environments when the intensity of light in their natural habitat increases.
When exposed to an intense burst of light, the blepharismin pigment will emit a poisonous toxin that will then disintegrate the organism. These released toxins are considered poisonous to humans and can in some cases be lethal. The process has been suggested by some to be a defense mechanism for Blepharisma japonicum.
In addition to the belief that the toxins released from the blepharismin granules may discourage any potential enemies, a recent discovery indicates that the mineral itself may be effective in combating Methicillin-Resistant Staphylococcus aureus (MRSA). This recent scourge among various hospitals, clinics, and other therapeutic locales may find this possibility quite effective in fighting off these prevalent infections. This will be discussed in more detail later.
To see this photophobic behavior for yourself, you can focus a strong light on a slide containing Blepharisma under the microscope. In a prolonged exposure the organisms will eventually be killed off. In natural surroundings this sensitivity to light functions as a biological safeguard to help keep the organisms at a productive place within their normal habitat. In other words, the Blepharisma is being warned to stay away from the upper layers of its aquatic environment where the sunlight is intense and to go deeper down where food sources are more likely.
Diet and Feeding
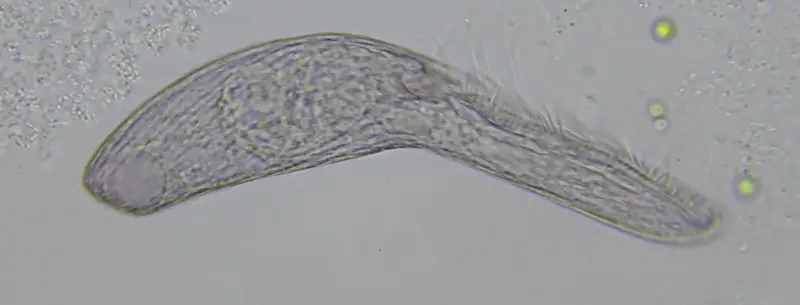
As with most of the protist family, Blepharisma tend to feed on a wide variety of smaller organisms being more of an opportunistic feeder than favoring any one type of diet. Blepharisma possess a full digestive tract and are classified as filter feeders. This simply means that they feed by straining their food that is suspended from the water using the cilia near the oral groove. Their food includes bacteria, flagellate algae (typically green algae in freshwater), rotifers, a variety of other ciliates, and sometimes, when the need arises, even smaller members of their own species.
On the subject of cannibalism, some experiments with Blepharisma have revealed a curious side note showing how nature often goes with the flow and will readjust when need be. In one species, Blepharisma undulans, there have been reports of gigantism seen when the organisms were forced to or proactively selected cannibalism.
When these individuals were offered a diet of smaller Blepharisma or even other ciliates ( e.g. Colpidium colpoda), they tended to grow to a relatively enormous size. For as long as their diet remained unchanged the putative “cannibal giants” underwent reproductive division to produce more and more of the same. However, if this type of prey were no longer available, then the subsequent offspring reverted to normal size.
For the truly curious, check out this video of the cannibalism process ( the whole video is a bit long so if you want to see the actual ingestion, skip over to about the 45-second mark):
Blepharisma Reproduction
Blepharisma reproduces in two ways. The first is an asexual method called binary fission and the second is a sexual method requiring more than one Blepharisma called conjugation. Binary fission is simply cytokinesis, or cell division, in which the organism divides along a transverse axis. Put simply, the original organism splits into two separate entities that resemble the original.
This process can occur spontaneously as part of the vegetative cell cycle or it can also follow the sexual variant, conjugation. The vegetative cell cycle is an event in which an original cell divides into two daughter cells. This event includes duplication of the DNA as well as some cellular organelles, with the subsequent partitioning of cytoplasm to each new daughter cell.
The cell cycle has two parts:
- Interphase: cell grows, replicates its DNA, and prepares for mitosis
- Mitotic phase (includes mitosis itself as well as cytokinesis): in mitosis, the replicated chromosomes are separated into new nuclei in preparation for cell division (cytokinesis)
Here is a short video clip of Blepharisma undergoing binary fission:
In conjugation, genetic material is exchanged between adjoined organisms as a temporary cytoplasmic conduit is formed between them. The macronuclei organelle of the cell provides housekeeping functions to the organism while the micronuclei are responsible for the transfer of genetic information during conjugation. During cell division and conjugation, the micronuclei move away from the macronucleus to the surrounding cytoplasm. The micronuclei of each organism undergoes meiosis.
Without going into great technical detail regarding this event, the simplest and most basic way to explain meiosis in Blepharisma conjugation is to know that multiple divisions occur within each organism that align to exchange genetic material. The divisions are referred to as reductive in nature as the genetic material is reduced in half before the exchange. This reduced form is known as haploid. Once all is recombined, the genetic material is referred to as diploid. The DNA is then reshuffled, and binary fission commences once the conjugation is complete. Half the material from each partner cell has now been exchanged to the other.

Conjugation itself centers around the discovery of gamones, chemical substances that have been used to induce conjugation by stimulating the conjugation interaction between partners. Ordinarily, conjugation occurs only with the interaction of cells of different mating types, but research has shown that clonal Blepharisma cells can be enticed to conjugate with one another. This clonal conjugation is sometimes referred to as “selfing”. In this regard it is an extreme version of inbreeding. Selfing can have obvious negative genetic consequences:
- Loss of genetic variation as heterozygote genes (gene locus when its cells contain two different alleles, one wild-type and one mutant ) become homozygous (gene locus contain identical alleles on both homologous chromosomes).
- Expression of harmful recessive alleles ( for simplicity purposes, assume an allele is a coding region of a gene).
Take Blepharisma japonicum as an illustrative example of the gamone phenomenon. In this species, there are two different mating types, I and II, with each one excreting pheromones specific to gamone I and gamone II. When type I cells become even moderately starved, they will automatically manufacture and then secrete gamone I, which acts on the mating cells of type II. The effect of this release is the transformation of type II cells so they can find and unite with type I cells. Simultaneously the type II cells produce and secrete gamone II which transform type I cells in a likewise manner. In this way, conjugation is enhanced, and sexual reproduction is advanced.
How to Culture Blepharisma
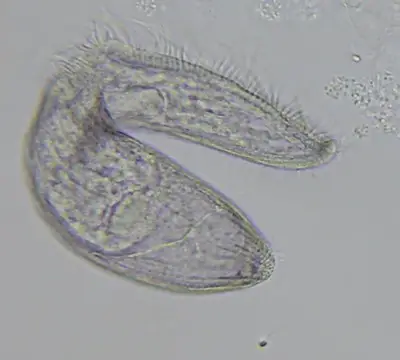
Culturing Blepharisma in the laboratory can be accomplished quite easily using an infusion of powdered cereal greens. The general culture medium that gave the best results in one study was using a product called Chlorovin, along with powdered organic grasses and an alfalfa additive. The one sensitive issue, though, is the water source used for making the medium.
In general, the condition of the water used in any protozoan culturing project is problematic. Tap water, even after boiling to remove chlorine, as well as distilled water can be toxic. This leaves the option of spring water or unpolluted water from a pond or stream, but the quality will vary here with the season and the source.
How to Observe Blepharisma Under the Microscope
For simple study, observation, and research, one may find the microscopic examination of Blepharisma a challenge as they tend to move about rather quickly at room temperature. The simple compound light microscope, found in most high school and university laboratories, is adequate for viewing and studying these organisms. The images in this post were captured using this microscope which I recommend for anyone that is interested in microscopy or microbiology.
A successful protocol has been reported by Janette Hanna while she was a student at the Rochester Institute of Technology as part of her coursework, “Principles and Techniques of Photomicrography” course in November 2004, to assist with this. In her methodology, she formed a circular ring of petroleum jelly on a microscope slide and then added Blepharisma specimens in water inside the ring. The jelly sealed in the water and still allowed the Blepharisma specimens room in which to move about.
She found that fresh specimens could survive using this tight jelly seal for over a week. To alleviate the problem of the organisms moving about too rapidly to observe and study, she found that placing the preparation in a refrigerator for several hours slowed their locomotion considerably. Other methods such as adding glycerin directly into the water with the specimens also helped to deter the rapid motion at room temperature but for her purposes, Janette found the refrigeration method most effective. If you are finding Blepharisma difficult to photograph with your microscope because they are moving too rapidly you can try this technique.
Blepharisma in Scientific Research
Blepharisma has, over time, been the subject and central focus of much scientific research. Part of it is obviously associated with the presence of the blepharismin granules while another is associated with the unique reproductive behavior that has been displayed. More attention has been garnished more recently on the so-called “cannibalistic” traits seen with various species as Blepharisma. Below you will find a number of interesting and fascinating research projects that involve Blepharisma.
Defense Function of Blepharismin against Two Predatory Protists
A research team in Japan investigated the defense function of blepharismin granules from Blepharisma japonicum against two separate protist predators, Amoeba proteus and Climacostomum virens. A comparison of normally-pigmented cells were compared to albino mutants. The normally-pigmented cells were less vulnerable to Amoeba proteus but not Climacostomum virens.
Blepharismin as an Antibiotic Effective Against Methicillin-Resistant Staphylococcus Aureus
A research team investigated the effectiveness of blepharismin from Blepharisma japonicum as a possible antibiotic treatment against Methicillin-Resistant Staphylococcus aureus (MRSA). Since first reported in 1961, MRSA has been a thorn in the side of doctors, nurses, and other clinical personnel in treating infections associated with this.
Even with two antimicrobial agents, arbekacin (ABK) and vanomycin having been moderately useful for the treatment of MRSA-related infections in Japan, the researchers knew a new agent would soon be needed. Their initial results indicated that the blepharismin isolated from Blepharisma japonica did indeed effectively inhibit the growth of an ABK-resistant MRSA strain.
Ultraviolet B Induction of Protection Strategy in Blepharisma
A research project from India looked at the effect that UV-B light would have on various species of Blepharisma and Notohymena in relation to the known fact that this wavelength of light is an environmental stress factor for a number of aquatic organisms. What was unknown at the beginning of the project was how this might impact freshwater ciliates and they were curious as to methods that these organisms might employ to withstand UV-B exposure.
Overall, they found that UV-B can lead to changes in cell morphology, reduced cellular movement, reduction in overall cell growth, formation of cysts, and increased UV absorption by the blepharismin granules. In response to these effects, the organisms in this study adopted various defense mechanisms such as encystment, change in the motility, and aggregation for survival strategies to the UV-B stress.
Chemical Defense of Blepharismin in New Species of Blepharisma
Based on the long-running knowledge of how Blepharisma japonica uses the five separate pigments known as blepharismins for both light perception and chemical defense against predators, the researchers here looked at two additional species to see if there was a similar effect. The new organisms selected were Blepharisma stoltei and Blepharisma undulans.
They purified the pigments of these new species and compared the toxicity of the purified proteins against a panel of ciliated protists and one metazoan predator. What they found was that the same chemical defense mechanism present in Blepharisma japonica occurred in the new species, mediated by the same five pigments but produced in different proportions.
Takeaways
Blepharisma is a fascinating microorganism to observe under the microscope not only for their distinct pink color but because watching them glide using their elongated cilia is mesmerizing. Their vacuoles are visible under about 400X magnification and it is easy to catch them in the middle of conjugation. If you ever come across Blepharisma take some time to appreciate this microorganism and take some great photos too!
Reference
- Giese, Arthur C. and Smith, Anne Muller, “Blepharisma in Introductory Biology”, The American Biology Teacher, October, 1973, p.4087.
- Giese, Arthur C. and Smith, Anne Muller, “Blepharisma in Introductory Biology”, The American Biology Teacher, October, 1973, p.407.
- Janette Hanna, Rochester Institute of Technology, 2005 (article posted on Micscape Magazine (Microscopy-UK).
- Hirshfield; et al. (1965). “A proposed organization of the genus Blepharisma Perty and description of four new species”. J. Protozool. 12 (1): 136–144.
- Miyake, Akio, Harumoto, Terue, Salvi, Bruna, and Rivola, Valeria, “Defensive function of pigment granules in Blepharisma japonicum”, European Journal of Protistology, Vol. 25, Issue 4, 29 June 1990, pg. 310-315.
- Giese, Arthur (1938). “Cannibalism and Gigantism in Blepharisma“. Transactions of the American Microscopical Society. 57 (3): 245–255.
- Giese, Arthur C. and Smith, Anne Muller, “Blepharisma in Introductory Biology”, The American Biology teacher, October, 1973, p.408.
- Lynn, Denis H. (2007). The Ciliated Protozoa: Characterization, Classification and Guide to the Literature (3 ed.). Springer. p. 23.
- Sugiura, M.; et al. (31 May 2005). “Developmentally and environmentally regulated expression of gamone 1: the trigger molecule for sexual reproduction in Blepharisma japonicum”. Journal of Cell Science. 118 (12): 2735–41.
- Miyake A. (1981). Cell interaction by gamones in Blepharisma In: Sexual Interactions in Eukaryotic Microbes (O’Day, D.H. editor), pp. 95-129 New York: Academic Press.
- Sugiura M, Shiotani H, Suzaki T, Harumoto T (2010). “Behavioural changes induced by the conjugation-inducing pheromones, gamone 1 and 2, in the ciliate Blepharisma japonicum“. Eur. J. Protistol. 46 (2): 143–9.
- Bernstein C, Bernstein H (1997). “Sexual communication”. J. Theor. Biol. 188 (1): 69–78.
- Terazima, Masayo Noda and Harumoto, Terue, “Defense Function of Pigment Granules in the Ciliate Blepharisma japonicum against Two Predatory Protists, Amoeba proteus (Rhizopodea) and Climacostomum virens (Ciliata)” , ZOOLOGICAL SCIENCE 21: 823–828 (2004), Zoological Society of Japan.
- Bijaya Pant, Yoji Kato, Takanori Kumagai, Tatsuomi Matsuoka, Masanori Sugiyama, “Blepharismin produced by a protozoan Blepharisma functions as an antibiotic effective against methicillin-resistant Staphylococcus aureus”, FEMS Microbiology Letters, Volume 155, Issue 1, October 1997, Pages 67–71.
- S. Sripoorna, Jeeva Susan Abraham, Swati Maurya, Hritik Kadian, Juhi, Jaiswal, Seema Makhija, Ravi Toteja, “Ultraviolet B Induced Protection Strategies in Unicellular Eukaryotic Microbes Blepharisma sp. and Notohymena sp. (Protista)”, DU Journal of Undergraduate Research and Innovation, Volume 3, Issue 1, pp 60-73.
- F. Buonanno, A. Anesi, G. Guella, and C. Ortenzi, “Blepharismins used for chemical defense in two ciliate species of the genus Blepharisma, B. stoltei and B. undulans”, The European Zoological Journal, Volume 84, 2017 – Issue 1, p. 402-409.