Macrophages (also known as leucocytes) are specialized white blood cells of the immune system and play a vital part in innate (inborn) immunity and immune responses of the body.
Macrophages were discovered in 1882 by Eli Mechnikoff and have been widely studied ever since. The word macrophage comes from the Greek meaning ‘large eater’.
This is because one of the most important roles of the macrophage is to phagocytose (ingest) other cells such as bacteria or parasites and large particles that may be harmful to the body. They also function as antigen presenters and produce substances called cytokines that aid communication in the immune system.
In this article you will learn everything you need to know about Macrophages and their structure, function, processes and more.
Macrophage Structure
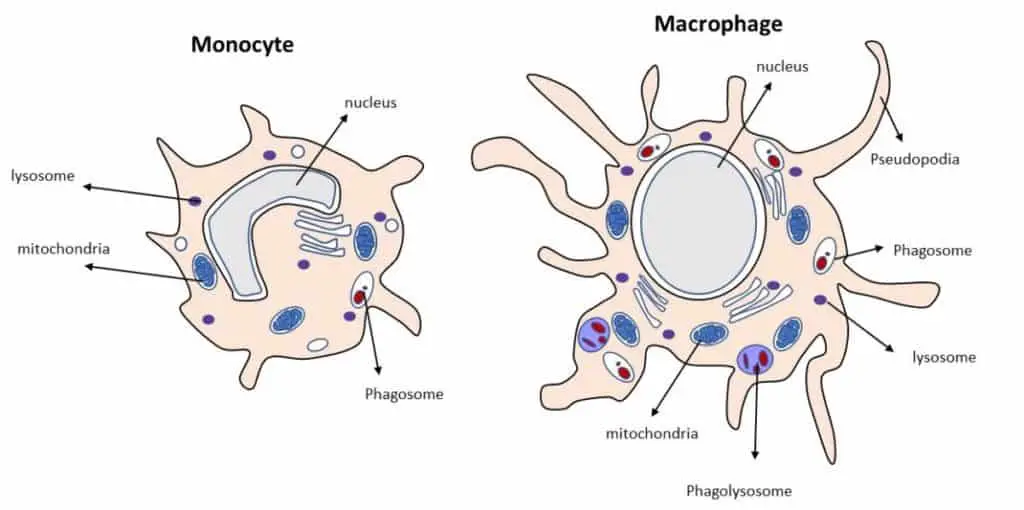
Monocytes are the largest type of white blood cell. Under a scanning electron microscope, it is a spherical cell with some protrusions in the cell membrane. When the monocyte moves into tissue and becomes a macrophage, it becomes even larger with more cytoplasmic granules.
The shape of the macrophage varies depending on the type of tissue it is in. Macrophages are approximately 21 micrometers in diameter. The video below shows a macrophage engulfing a chain of bacteria.
Monocytes have a kidney-shaped nucleus. The cytoplasm is full of mitochondria as well as microtubules and microfilaments. A large quantity of mitochondria helps to power the cells and the microtubules and microfilaments help with cell structure as part of the cell cytoskeleton.
Near the cell surface, there are many microvilli, helping to increase the cell surface area. Microcytotic vessels are also found near the surface of the cell. The cytoplasm has granules like lysosomes, which contain enzymes that help to destroy engulfed pathogens.
There are many cell receptors present on the macrophage. These include complement proteins, cytokines, chemokines, Fc receptors (which bind to antibodies), and lipoproteins as well as many others.
When the monocyte differentiates into a macrophage within a tissue, the number of granules increases, as does the cell volume. Once they have differentiated, the macrophage shape and structure depend on which tissue it resides.
All macrophages, however, have membrane-bound lysosomes. These fuse with phagosomes and form what is known as secondary lysosomes. These secondary lysosomes contain material that has been ingested. Finally, another characteristic of the macrophage is its pseudopodia.
Pseudopodia are arm-like projections filled with cytoplasm and rich in the protein actin which helps hold the shape of the pseudopodia. These projections aid with the motion of the macrophage and ingestion of foreign materials.
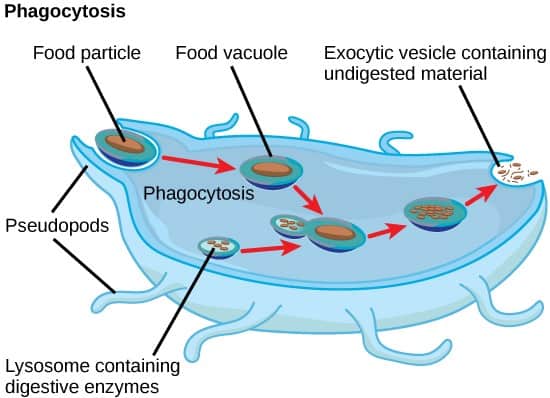
Phagocytosis (Cell Eating)
Phagocytosis is described as a form of endocytosis. This process involves taking up of particles through the invagination of the cell membrane.
Phagocytosis is a specific form of endocytosis that involves the use of cell surface receptors necessary for taking up specific particles. Phagocytosis allows the ingestion of microbial pathogens, but also apoptotic cells (dying cells).
Therefore, they are not just important in defense against microbes, but also in tissue homeostasis. The process of phagocytosis can be broken down into 4 steps.
Step 1: Step one begins with the recognition of a particle on a binding site receptor on the surface of the macrophage.
Step 2: This binding triggers the remodeling of the cytoskeleton by the formation of an actin-rich extension of the cell membrane. Actin is an important structural protein, and its distribution affects the shape of the cell membrane. This extension invaginates the particle.
Step 3: The membrane fuses and forms a vacuole known as a phagosome.
Step 4: The phagosome fuses with a lysosome to give rise to a phagolysosome. For more on lysosomes check out this post. It has a low pH for optimal function of its enzymes. The contents of the lysosome go into the phagosome. Organisms are destroyed within the phagolysosome.
The process of phagocytosis is part of the innate immune system. The molecules that have been taken up by the macrophage can then be exported out of the cell or presented on their cell surface.
This is known as antigen presentation, which allows other cells of the immune system to be activated leading to acquired immunity. In this way, the innate immune system and the acquired immune system can link together.
Opsonic vs Non-Opsonic Phagocytosis
Receptors on the cell membrane can be described as either opsonic or non-opsonic. Opsonic receptors recognize host-derived opsonins that have bound to foreign particles. Opsonic receptors mark dead or dying cells. Opsonins include antibodies, complement proteins, the glycoprotein fibronectin, and mannose-binding lectin.
The most important phagocyte opsonic receptors are the Fc receptors (antibody binding receptors) and the complement receptors. For example, antibodies that bind to the microbe will then attract phagocytes such as macrophages.
Non-opsonic phagocytosis is triggered by cell surface receptors on the macrophage that recognize molecular patterns on microbes themselves. CD169 and CD 33 are examples of receptors for these molecular groups.
How Do Macrophages Develop?
Macrophages are a type of monocyte which can be generated from cells in the bone marrow called hematopoietic stem cells. This process is known as hematopoiesis. Stem cells are able to differentiate into various types of blood cells.
The origin of macrophages has been the subject of debate over the last 30 years. It is currently thought that most tissue macrophages are planted in the very early embryo before the process of hematopoiesis is established.
These tissue-resident macrophages may persist in tissues during adult life as it is thought that they self-renew. At birth, hematopoietic stem cells constantly replenish the blood cells circulating in the bloodstream in response to demand.
Therefore, macrophages found in different tissues may be a mix of embryo-derived or bone marrow-derived macrophages. The origins of the macrophage and its precursor cells are still under investigation by scientists today.
The generation of macrophages from hematopoietic stem cells is a complicated process. These cells can differentiate into either myeloid precursors or lymphoid precursors. Macrophages result from a common myeloid progenitor or myeloid cell.
Other lymphocytes such as natural killer cells, B and T lymphocytes develop from a common lymphoid progenitor or lymphoid cell. The figure below shows the differentiation of hematopoietic stem cells. As we are interested in the development of the macrophage, we will follow the myeloid progenitor pathway.
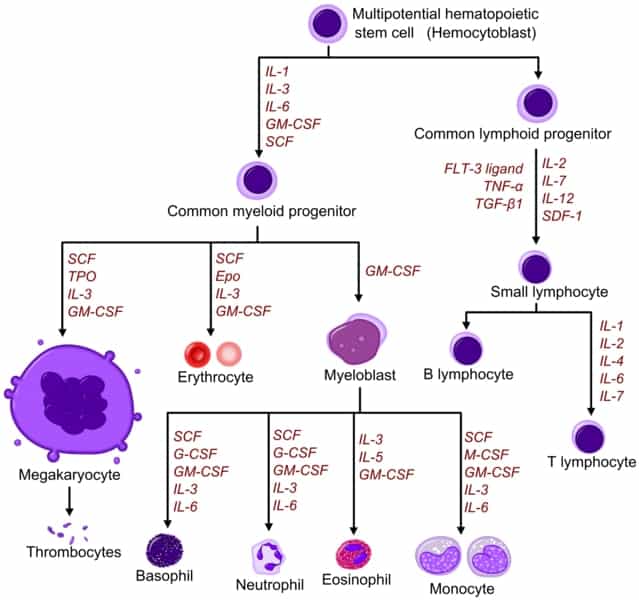
The myeloid progenitor pathway begins with the myeloid progenitor cell migrating into the bloodstream. It then differentiates into a monocyte. Blood monocytes circulate the blood system for around 1 to 3 days before they mature into tissue macrophages.
In body tissue, they can live for several months. If there is tissue damage in the body, a monocyte leaves the blood system to the site of damage where it undergoes chemical changes to become a tissue macrophage.
The differentiation of monocytes to macrophages is dependent on certain chemicals, particularly cytokines. Cytokines can be described as a group of proteins, glycopeptides, or peptides involved in the regulation of the immune system. Cytokines regulate and mediate immunity, inflammation, and hematopoiesis.
Monocytes are described as heterogeneous, meaning there are variations in their characteristics. Most human monocytes are called ‘classical’ monocytes. These are differentiated by the expression of CD antigen molecules.
The term ‘CD’ stands for ‘cluster of differentiation’ and they are molecules (also described as antigens) expressed on the cell surface of white blood cells. They are commonly used in cell sorting methods to distinguish different types of white blood cells within a sample.
To date, there are over 400 documented CD molecules. Classical monocytes make up most of the monocyte population. These cells express CD14, but not CD16. The remaining proportion of monocytes are divided into 2 subsets, these are called the intermediate subset and the non-classical monocytes.
Intermediate monocytes express CD14 in high amounts and low amounts of CD16. Non-classical monocytes have a high level of CD16 expression and a low level of CD14 expression.
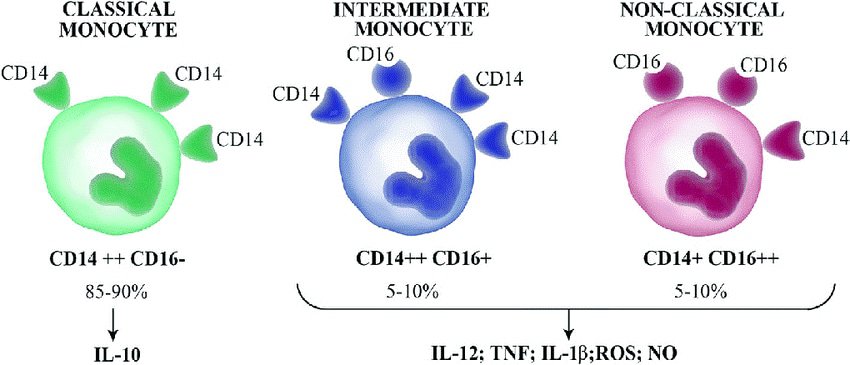
Classical monocytes are involved in inflammatory responses and phagocytosis. These are the monocytes that differentiate into macrophages in body tissues and have high phagocytotic activity. Dendritic cells also derive from classical monocytes.
Intermediate monocytes have the potential to differentiate into macrophages or dendritic cells. They go on to stimulate T cell production, are involved in inflammatory responses, erythropoiesis (the development of new blood vessels), and cell differentiation.
Non-classical monocytes usually differentiate into macrophages and are involved in viral responses and patrolling for sites of injury or damage. They secrete cytokines in response to infection and have pro-inflammatory behavior.
Monocytes To Macrophages – How Does This Happen?
Monocytes are stimulated to become macrophages in response to a set of cytokines called colony-stimulating factors (CSF). M-CSF or (CSF1) and GM-CSF (CSF2) are involved in specific macrophage differentiation.
Macrophage cells induced by GM-CSF are named M1 macrophages and are involved in antigen presentation and inflammation. M-CSF induced cells are named M2 macrophages and have the role of phagocytosis and release of a cytokine called interleukin-10.
The process by which macrophages adapt their functions and characteristics (phenotypes) in response to their environment is termed macrophage polarization. M1 macrophages are described as being classically activated whereas M2 macrophages are described as being alternatively or non-classically activated. M2 macrophages are further classified into M2a, M2b, and M2c. The induction routes of these polarized macrophages are incredibly complex but will be summarized in the next few paragraphs.
Full macrophage activation is thought to require their previous priming by low levels of different types of the cytokine interferon (IFN). When interferon-gamma (IFN- γ), GM-CSF and/or lipopolysaccharide found in bacteria are presented, M1 macrophages are activated. These M1 macrophages then produce a high level of inflammatory cytokines.
Tumor necrosis factor-alpha (TNFα), interleukin 6 (IL-6), and interleukin 12 (IL-12) levels are increased. The release of these cytokines enables M1 macrophages to act as the first line of defense against intracellular pathogens. These macrophages also remove tumor cells, control tissue damage, and prevent wound healing due to their role in inflammation.
M2 macrophages are activated differently. They differentiate under the influence of interleukin 4 (IL-4) and interleukin 13 (IL-13). These cytokines are produced by a subset of T cells known as T-helper 2 cells (Th2). As mentioned earlier, M2 cells are further differentiated. M2a are induced by the cytokines IL-4 or IL-13. M2a cells are involved in the removal of parasites and promote fibrosis.
M2b macrophages are induced by immune complexes, toll-like receptors (TLRs), IL-10, or interleukin 1 receptor agonist (IL-1ra). They are immune regulating cells. M2a and M2b macrophages both help to enhance Th2 responses. Finally, M2c cells are activated by interleukin 10 (IL-10) and it is thought that they are deactivated or anti-inflammatory in nature and are involved in immune suppression and tissue remodeling.
Macrophage Locations In The Body
Macrophages are named differently depending on their location. For example, macrophages found in the liver are called Kupffer cells, in the brain, they are known as microglia, and in the lungs, they are known as alveolar macrophages.
| Macrophage Type | Location | Function |
|---|---|---|
| Alveolar macrophages | Alveoli in the lungs | Phagocytosis and immunity to pathogens, dust, allergens, and other particles |
| Kupffer cells | Liver | Liver tissue repair, clearance of pathogens and toxins |
| Microglia cells | Central nervous system | Immunity of the brain, brain development, and removal of old neurons |
| Splenic macrophages Red pulp macrophages Marginal zone macrophages | Spleen Red pulp Marginal sinus | Removal of dead or old (senescent) red blood cells Removal of blood-borne pathogens |
| Intestinal macrophages | Intestine | Removal of gut pathogens and tolerance to food antigens and gut flora |
| Lymph node macrophages | Lymph nodes | Antigen presentation to B cells to allow antibody generation. This activates the adaptive immune system. |
| Monocytes | Blood | Patrolling |
| Dermal macrophages | Dermis (skin) | Patrolling |
| Kidney macrophages | kidney | Patrolling |
| Connective tissue macrophages | Connective tissue | Tissue macrophages leading to giant cells (a mass of multiple cells). Giant cells are formed following inflammation |
| Osteal macrophages | Bone | Regulate bone formation and homeostasis |
What Are The Functions Of Macrophages?
As mentioned above, macrophages have a wide variety of activities within the immune system. Let us have a look at some of these important roles in more detail.
Bone Remodeling
Macrophages have been observed during all stages of bone fracture healing. Macrophages in the bone tissue are termed osteal macrophages. Their phagocytic ability allows them to clear up dead cells and allow the recruitment of new cells to maintain homeostasis.
M2 macrophages have been found during the process of ossification (laying down new bone during repair). This implies they have an important role in the success of bone regeneration. This area of immunology is known as osteoimmunology and the specific mechanisms that macrophages use for bone homeostasis and repair are still undergoing research.
Erythropoiesis
Erythropoiesis is the process of the differentiation of hematopoietic stem cells into red blood cells. Macrophages provide signals that induce erythropoiesis. Macrophages surround developing red blood cells and provide nutrients such as iron.
They also phagocytose nuclei that are expelled by red blood cells. It is thought that macrophages can remove dead or abnormal red blood cells by recognizing cell surface markers which they can bind with their scavenger receptors. An example of such a receptor is CD36. This is triggered by specific signals expressed on the surface of the red blood cells.
Brain Development
Macrophages in the central nervous system are termed microglia. Microglia are found in the developing brain. Here, they have a role in phagocytosis, synaptic pruning (a phase in the development of the nervous system), and neural protection.
This area is still very unknown, and more studies need to be carried out on the human brain to determine exactly how macrophages are involved in these processes. It has also been suggested that they may be involved in diseases such as schizophrenia and Alzheimer’s.
Lung Homeostasis
Alveolar macrophages have an important role as the first line of defense against incoming pollutants and microbes. M1 and M2 macrophages are present in the lung tissue. The M1 macrophages phagocytose microbes and contribute to inflammation, which increases blood flow to the area, enhancing microbial destruction by the recruitment of immune cells.
M2 cells on the other hand have an anti-inflammatory function and are involved in phagocytosis of apoptotic (or dying) cells. They are also involved in the repair of damaged tissue. Therefore, these subsets of macrophages act together encouraging homeostasis of the lung tissue area.
Iron Recycling
Macrophages that are involved in iron recycling include red pulp macrophages in the spleen, Kupffer cells in the liver, and macrophages derived from the bone marrow. Macrophages can recycle around 90% of the body’s iron.
They do this via a variety of receptors binding either free-iron or iron-bound molecules. After uptake, the iron, or iron-bound protein is processed and then either stored, used in cellular processes, or released into the circulation. A surplus of iron is stored in hepatocytes and macrophages as ferritin. Ferritin is a protein that can contain up to 4,500 iron molecules.
Iron stored within macrophages is used by a range of proteins as a cofactor that enables the regulation of energy production, hypoxic regulation, and detoxification. They also regulate host defenses and inflammation.
Red blood cells have a lifespan of around 120 days. When they reach the end of their life, they become trapped inside the spleen. Here, they are recognized by red pulp macrophages and destroyed. The hemoglobin of the red blood cells is recycled and then iron is exported into sinusoidal capillaries. Here, it is bound to the glycoprotein transferrin.
This iron can then be used by developing red blood cells (erythroblasts). These erythroblasts are found surrounding a macrophage, this macrophage is termed an erythroid island macrophage. Here they assist with the differentiation of the red blood cells and iron uptake.
The amount of iron in plasma is detected by hepatocytes (liver cells). These cells aid with the regulation of iron transfer involving the hormone hepcidin. Kupffer cells (liver macrophages) inhibit the expression of hepcidin by hepatocytes and carry out phagocytosis of red blood cells.
Tissue Regeneration, Repair, and Fibrosis
Tissue macrophages are involved in the repair, regeneration, and fibrosis of tissue. Following injury, macrophage precursor cells from the bone marrow are directed to the injured tissue site by cytokines. These macrophages along with the tissue macrophages already present, begin to proliferate and undergo changes in response to their chemical environment.
If the injury is severe, macrophages can activate neighboring cell populations to become involved in the repair. The macrophages function to stimulate the proliferation, differentiation, and activation of several different cell types. These include fibroblasts, endothelial cells, epithelial cells, and stem cells which are all involved in the repair.
In the final stage of repair, macrophages change their phenotype to control any further inflammatory responses that may damage tissue. This is a switch from pro-inflammatory macrophages to anti-inflammatory macrophages. Their importance is shown when the process is not controlled, and the destruction of tissue is seen via fibrosis (thickening and scarring of tissue).
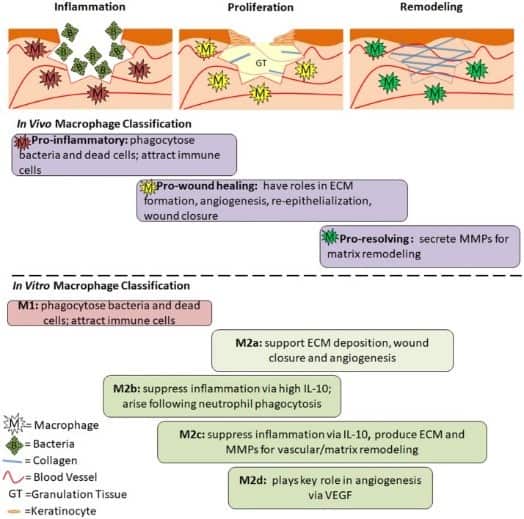
The tropical parasite Leishmania can exploit wound healing macrophages. Leishmania causes skin sores and ulcers, an enlarged spleen, and liver damage. It can decrease the immunity of the host leaving them vulnerable.
Some individuals recovering from this disease have immune systems that go ‘haywire’ and create an inflammatory response making their symptoms worse, i.e. wounds take longer to heal. Leishmania can enter the macrophage and survive within it, making it difficult for the immune system to clear.
Thermogenesis
Thermogenesis is the production of heat in warm-blooded animals. In animals, there are 2 types of fat, white adipose tissue, and brown adipose tissue. White adipose tissue stores energy in the form of triglycerides (constituents of fat) and brown adipose tissue transfers energy from food into heat.
Brown adipose tissue dissolves energy to produce heat and regulates the balance of energy. Brown adipose tissue is more energy productive. It is thought that brown adipose tissue may protect against obesity. M2, non-pro-inflammatory macrophages are involved in promoting brown adipose tissue thermogenesis.
Macrophages And Obesity
Chronic low-grade inflammation is seen in the white adipose tissue of obese individuals as well as insulin resistance. Macrophages are important in maintaining adipose tissue homeostasis. When obesity occurs, there is an increase in pro-inflammatory mediators that leads to a switch in the phenotype of an M2 macrophage to an M1 macrophage.
This increase in proinflammatory cytokines recruits other cells of the immune system including the number of macrophages. In turn, this then produces more proinflammatory mediators and chronic inflammation ensues. Insulin resistance is a reduction in the liver, muscle, and adipose tissue to insulin.
Obesity is associated with insulin resistance and the onset of type 2 diabetes. It is thought the switch from the M2 macrophage to the M1 macrophage powering chronic inflammation may be a cause of impaired insulin signalling. The cytokines TNF-α and IL-6 are pro-inflammatory cytokines found in higher levels where insulin resistance is recorded.
Conclusion
Macrophages have a wide range of vital roles in the human body, they are not just a part of the innate immune system as the first line of defense in the innate immune system. They have a wide range of roles from brain development to tissue repair.
The pathways involved in these various processes are incredibly complex, and still being investigated today. Their importance in the body can be observed when they do not function correctly, as in certain parasitic infections, leading to prolonged and damaged healing and fibrosis.
References
- Castoldi, A., et al. (2016) The Macrophage Switch in Obesity Development. Front. Immunol. https://doi.org/10.3389/fimmu.2015.00637
- Elhelu M. A. (1983). The role of macrophages in immunology. Journal of the National Medical Association, 75(3), 314–317.
- Engel, P., et al. (2015). CD Nomenclature 2015: Human Leukocyte Differentiation Antigen Workshops as a Driving Force in Immunology. The Journal of Immunology 195 (10) 4555-4563. https://doi.org/10.4049/jimmunol.1502033
- Jagannathan-Bogdan, M., & Zon, L. I. (2013). Haematopoiesis. Development (Cambridge, England), 140(12), 2463–2467. https://doi.org/10.1242/dev.083147
- Gordon, S., & Martinez-Pomares, L. (2017). Physiological roles of macrophages. Pflugers Archiv : European journal of physiology, 469(3-4), 365–374. https://doi.org/10.1007/s00424-017-1945-7
- Gordon, S., Plüddemann, A. Tissue macrophages: heterogeneity and functions. BMC Biol 15, 53 (2017). https://doi.org/10.1186/s12915-017-0392-4
- Hu, G., & Christman, J. W. (2019). Editorial: Alveolar Macrophages in Lung Inflammation and Resolution. Frontiers in immunology, 10, 2275. https://doi.org/10.3389/fimmu.2019.02275
- Klei, T. R., Meinderts, S. M., van den Berg, T. K., & van Bruggen, R. (2017). From the Cradle to the Grave: The Role of Macrophages in Erythropoiesis and Erythrophagocytosis. Frontiers in immunology, 8, 73. https://doi.org/10.3389/fimmu.2017.00073
- Macrophage Phagocytosis Assay. Creative Bioarray. https://www.creative-bioarray.com/support/macrophage-phagocytosis-assay.htm
- Mandal, A. What is a Macrophage? News Medical Life Sciences. https://www.news-medical.net/life-sciences/What-is-a-Macrophage.aspx#:~:text=Macrophages%20are%20formed%20through%20the,of%20changes%20to%20become%20macrophages.
- Menassa, D.A., Gomex-Nicola, D. (2018). Microglial Dynamics During Human Brain Development. Front. Immunol. https://doi.org/10.3389/fimmu.2018.01014
- Muraille, E., Leo, O., & Moser, M. (2014). TH1/TH2 paradigm extended: macrophage polarization as an unappreciated pathogen-driven escape mechanism?. Frontiers in immunology, 5, 603. https://doi.org/10.3389/fimmu.2014.00603
- Nairz, M., Theurl, I., Swirski, F. K., & Weiss, G. (2017). “Pumping iron”-how macrophages handle iron at the systemic, microenvironmental, and cellular levels. Pflugers Archiv : European journal of physiology, 469(3-4), 397–418. https://doi.org/10.1007/s00424-017-1944-8
- Narasimhan, P.B., et al. (2019) Nonclassical Monocytes in Health and Disease. Annual Review of Immunology. Vol. 37:439-456 https://doi.org/10.1146/annurev-immunol-042617-053119
- Orekhov et al. (2019). Monocyte Differentiation and Macrophage Polarization. Vessel Plus. 3:10 https://oaepublishstorage.blob.core.windows.net/c97586be-2680-45ca-b519-edaf65e80d30/3016.pdf
- Sampath, P. 2018. Monocyte subsets: Phenotypes and Function in Tuberculosis Infection. Front. Immunology. https://doi.org/10.3389/fimmu.2018.01726
- Remmerie, A., & Scott, C. L. (2018). Macrophages and lipid metabolism. Cellular immunology, 330, 27–42. https://doi.org/10.1016/j.cellimm.2018.01.020
- Rosales, C., & Uribe-Querol, E. (2017). Phagocytosis: A Fundamental Process in Immunity. BioMed research international, 2017, 9042851. https://doi.org/10.1155/2017/9042851
- Shlundt, C., et al. (2018). Macrophages in Bone Fracture Healing: Their Essential Role in Endochondral Ossification. Bone. (106) 78-89. https://doi.org/10.1016/j.bone.2015.10.019
- Sukhbaatar, N., & Weichhart, T. (2018). Iron Regulation: Macrophages in Control. Pharmaceuticals (Basel, Switzerland), 11(4), 137. https://doi.org/10.3390/ph11040137
- Villarroya, F., et al. (2018) Toward an Understand of How Immune Cells Control Brown and Beige Adipobiology. Cell Metabolism. 27 (5) 954 – 961. http://doi.or/10.1016/j.cmet.2018.07.006
- Williams, J. W., Giannarelli, C., Rahman, A., Randolph, G. J., & Kovacic, J. C. (2018). Macrophage Biology, Classification, and Phenotype in Cardiovascular Disease: JACC Macrophage in CVD Series (Part 1). Journal of the American College of Cardiology, 72(18), 2166–2180. https://doi.org/10.1016/j.jacc.2018.08.2148
- Yao, Y., Xu, X. H., & Jin, L. (2019). Macrophage Polarization in Physiological and Pathological Pregnancy. Frontiers in immunology, 10, 792. https://doi.org/10.3389/fimmu.2019.00792