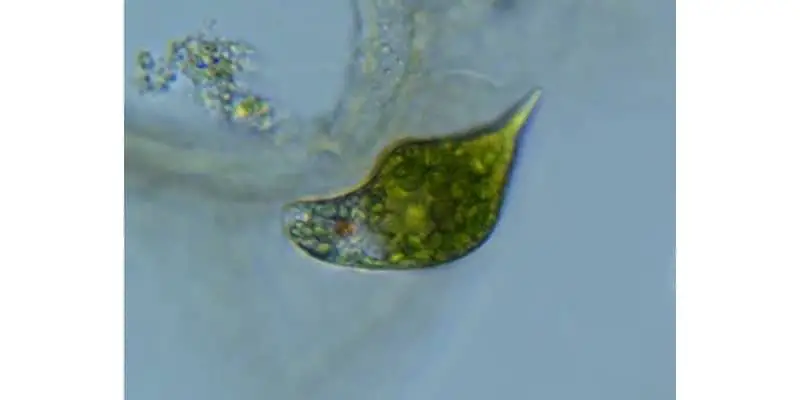
The microscopic world is humongous and hidden in plain sight. There are millions of organisms with a story to tell. Euglena is one genus that belongs to this extraordinary world.
Euglena is a genus made up of single celled eukaryotic organisms ranging in size from 15 to 500 micrometers whose characteristic green color stems from the presence of chloroplasts which enables Euglena to produce energy from photosynthesis. The Euglena genus are made up of over 800 species and are also known for their shape shifting abilities, whipping flagella, and the famous red eyespot called stigma.
Euglena were one of the first organisms seen under the microscope. The species Euglena gracilis has been extensively studied by scientists as a model organism, especially of the stress-response machinery (Watanabe & Suzuki 2004). This genus was described by Ehrenberg in 1830, as unicellular eukaryotes with chloroplasts, contractile vacuole, a red eyespot and two flagella.
Although Euglena are commonly thought of as the scum floating on the top of the water, in this article we will go through some amazing facts and history that should garner a greater appreciation for these microorganisms.
Euglena Structure and Anatomy
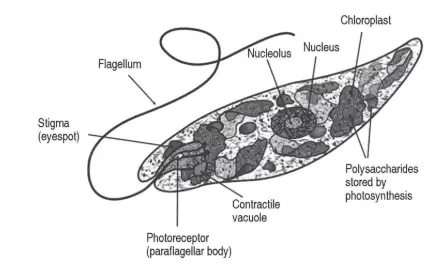
Euglena have an elongated shape, with lengths from 15 to 500 micrometers (to put this into perspective 1 cm is 10000 times a micrometer). This genus has certain characteristics that separate them from other groups, such as chloroplasts surrounded by three membranes where plants and green algae have two membranes and chloroplasts containing pyrenoids that synthesize paramylon (a carbohydrate similar to starch), which is unique for this genus.
Instead of having a cell wall, Euglena have a pellicle made up of a protein layer which has a striped pattern and surrounds the cell (helping to keep its shape) (figure 1). In fact, the flexibility and contractility of Euglena it’s given by these pellicle stripes sliding among each other (Sánchez et al. 2004, Singleton 2018).
Like other genera from the Euglenozoa phylum, Euglena has a red eyespot, an organelle that contains carotenoid pigments and filters the sunlight that is received by the photoreceptor structure located on the base of the flagellum (the paraflagellar body) responsible of directing the cell movement.
Euglenoids also have 2 flagella (whip-like structures) at the anterior end of the cell. One is short and non-emerging and the other is long and emerges from the cell. The longer flagellum is used primarily for locomotion and in some cases, helps to create a current which brings food particles closer (Singleton 2018).
What do Euglena Eat?
Most Euglena species have the ability to be both heterotrophic and autotrophic. Heterotrophs can be feed on bacteria, microscopic eukaryotes, and dissolved organic compounds and autotrophs used their photosynthesizing chloroplasts. An interesting fact is that when light is not available most species of Euglena have the ability to become heterotrophic (Zakryś et al. 2017, Singleton 2018).
Euglena Reproduction
Euglena reproduces asexually by binary fission and there’s no evidence of sexual reproduction. In binary fission the nucleus of the parent cell divides by mitosis (part of the cell cycle where DNA is replicated and chromosomes are separated into two nuclei), then the cell begins to divide at the anterior end of the cell, with the duplication of the flagella forming a V shape which gets bigger as it reaches the posterior end of the cell (Singleton 2018).
Euglena Habitat
Species of Euglena can be found in saltwater, freshwaterand moist soils, sometimes forming blooms (accumulation and population growth of algae) on ponds and lakes that color the surface of red or green and is visible to the naked eye.

Euglena can be found in a wide range of conditions, this is due to their ability to survive, actually when environmental conditions are adverse Euglena surrounds itself by a protective wall and forms a dormant stage called cyst that permits the survival of the organism until the environmental conditions improve. This strategy is common among other unicellular organisms (Fryxell 1983, Radzikowski 2013).
Interesting discoveries have revealed a high ability of some Euglena species to survive extreme conditions, for example, Euglena pailanensis is thermotolerant, surviving temperatures near 45°C and Euglena mutabilis tolerant to high metals and acid conditions it’s capable to grow at a pH of 1.3. Also, Sittenfeld and other authors discovered in 2002 a Euglena strain in an acidic hot mud pool near a Costa Rica’s volcano, facing temperatures between 35 to 98 °C and pH between 2 to 4 (Sittenfeld et al. 2002, Sánchez et al. 2004).
History and Scientific Classification
Antonie Van Leeuwenhoek was a Dutch scientist known as the father of microbiology. He designed and tested microscopes using magnifying lenses and was the first to document observations of bacteria, red blood cells, spermatozoa, muscle fibers, among others. For more on the history microscopes see The First Microscope to Modern Microscopes: Evolution and History of Microscopes.
The results of his observations were sent to the Royal Society (United Kingdom’s national academy of sciences) and in 1673 they published a letter from Van Leeuwenhoek with the observations on mold, bees and lice (Dobell 1923). In 1674 he wrote a letter to the Royal Society describing microbes with similar characteristics of Euglena viridis collected in water samples from an inland lake. Therefore, this Euglena species is among the first organism observed under the microscope (Singleton 2018).
In 1696 John Harris published microscopic observations describing an organism similar to Euglena, oval shape, with the middle part green and each end transparent and capable of contract and dilate themselves.
Ninety years later, Otto Muller named the organism with these characteristics Cercaria viridis and published illustrations of Euglena’s body.
Finally, Christian Ehrenberg in 1830 renames Muller’s Cercaria as Euglena, choosing this name from the Greek [eu] ‘well, good’ and [glene] ‘eyeball, socket join’ after describing Euglena’s eyespot as a rudimentary eye (Singleton 2018). Ehrenberg grouped in this genus four species already described and identified many new Euglena species, his illustrations are available at the Museum for Natural History in Berlin and we can found some Euglena’s drawings between the images 546 and the 559, like the one in figure 2 (Zakryś et al. 2017).
For more than 150 years scientists identified Euglena species based only on their morphological characteristics and the genus served as a bucket where all the species that didn’t fit in other genera were discarded, as a result, the genus was highly heterogeneous and the correct identification was difficult to verify.
The main morphological diagnostic features to identify euglenids were the morphology and organization of pellicular stripes, chloroplast morphology, presence of pyrenoids, morphology of the paramylon grains and presence and shape of mucocysts (small organelles located under the pellicle) (Zakryś et al. 2017).
With the development of molecular phylogenetics, there was a major reorganization of the Euglena genus. Molecular phylogenetics allow us to identify evolutionary relationships using molecular markers (a specific part of the DNA with a known location within the genome), the first phylogenetic analysis of Euglena species was based on nuclear small subunit ribosomal DNA (nSSU rDNA) (the most commonly used molecular marker) (Montegut-Felkner & Triemer 1997, Zakryś et al. 2017).
Over time, phylogenetic analyses increased, and additional molecular markers were employed such as internal transcribed spacer (ITS), nuclear and chloroplast large subunit of ribosomal DNA, cytoplasmic small subunit (cpSSU rDNA) and protein-coding sequences.
These analyses were essential to the reorganization of the genus, among the major changes were the reclassification of 1. Two Euglena species to a new genus, Discoplastis, 2. One Euglena species to a new genus, Euglenaformis, and 3. Three Euglena species to a new genus, Euglenaria (Zakryś et al. 2017).
The phylogenetic tree constructed after these studies was paraphyletic (where a taxon –a taxonomic group of any rank- doesn’t include all the descendants of the most common ancestor) and polyphyletic (where a taxon descended from more than one ancestor) with two species branching out of the main trunk of the genus Euglena archaeoplastidiata and Euglena velata (figure 3) (Ashlock 1971, Zakryś et al. 2017).
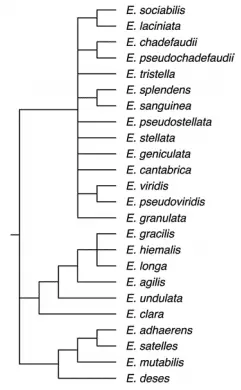
The current taxonomic hierarchy of the Euglena genus according to Ruggiero and collaborators in 2015, is the following: Superkingdom Eukaryota, Kingdom Excavata, Phylum Euglenozoa, Subphylum Euglenoida, Class Euglenophyceae, Order Euglenida and Family Euglenaceae.
Potential Uses of Euglena
Microalgae are interesting organisms for applied research and commercialization because of their high nutritional value containing vitamins, minerals, proteins, polyunsaturated fatty acids, antioxidants, among others. Euglena gracilis is a promising alternative supplement because it’s a source of dietary protein, pro vitamins, lipids and the β-1,3-glucan paramylon, in fact, studies have demonstrated that microalgae biomass can be a potential substitute for soybean and fish meal (Aemiro et al. 2016, Gissibl et al. 2019).
Based on this species characteristics and its ability to synthesize diverse and unique bioproducts, Gissibl and collaborators presented a summary on the industrial potential of Euglena gracilis (figure 4) (Gissibl et al. 2019). There is a strong development on Euglena gracilis bioproducts, which has been reflected in the establishment of new companies that specialized in Euglena cultures.
The cultivation method has a major impact on the total protein content, photoautotrophic cultivation of Euglena is the most common method, probably because the low cost and easy management, with a maximal yield of 0.5 g/g DW, while heterotrophic cultivation it’s more expensive and capable of providing higher biomass and paramylon yield, close to 0.7 g/g DW, and their industrial potential could become a reality in the near future. Although biofuel production is not yet possible, the improvement on carbohydrate and lipid yields of Euglena gracilis could become an affordable alternative to fossil fuels. (Gissibl et al. 2019).
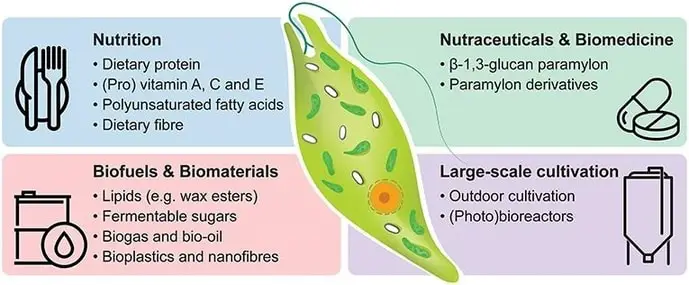
The benefits of using Euglena bioproducts are not limited to those aforementioned. Euglena gracilis can also be used to enhance the nutritional value of animal feed and increase the absorption of CO2 emissions in the atmosphere through carbon dioxide fixation. This is extremely important because can mitigate the effect of greenhouse emissions and therefore, the impact of climate change.
To investigate the effect of Euglena on CH4 emissions, Aemiro and collaborators tested different concentrations of Euglena in animal feed and found a decrease in methane emission by 9-48% with concentrations of 100 g/kg DM (dry matter). This concentration of Euglena gracilis also improved the digestibility of dry and organic matter of the animal (Aemiro et al. 2016).
In summary, if these lines of research are continued, it could possibly lead to many key developments including the improvement of food nutritional value for human consumption and livestock, the potential development of biofuels and mechanisms to mitigate the impact of climate change, especially with the decrease of CH4 emissions.
Are Euglena Harmful?
One species of the Euglena genus has been reported as toxic. Euglena sanguinea produces an alkaloid toxin similar to the fire ant venom called euglenophycin (Zimba et al. 2017).
In 2004, Zimba and collaborators reported unexplained fish mortalities at an aquaculture facility in North Carolina, over 21,000 striped bass died with no specific pathology. When other species were exposed to the pond water the fishes were killed in less than 7 minutes. Euglena sanguinea was isolated and identified after microscopic analysis from the water samples of the pond, to prove their toxicity juvenile catfish were exposed to the isolated alga (with a density of 1220 cells/milliliter) and died after 2 hours of exposure (Zimba et al. 2004).
In 2010, eleven additional events in freshwater aquaculture systems were reported, and fish exposed to euglenophycin died, included catfish, tilapia, sheepshead and striped bass, causing losses over the $1,100,000.
In this study, scientists also tested the activity of the toxin against other algae and against two colon cancer human cell lines and found that this toxin inhibits phytoplankton growth and tissue cell growth in mammalian cancerous cell lines (anticancer activity) at low parts per million dosages (Zimba et al. 2010).
Regarding the anticancer activity, Zimba and collaborators tested the effect of euglenophycin concentrations on three different human leukemias and found that euglenophycin reduced the number of viable leukemia cells (up to 50% with a toxin concentration of 25 micrograms/milliliter and 100% with a concentration of 100 micrograms/milliliter after 48 hours of treatment) and their metabolic activity in vitro.
This suggested that euglenophycin could be used in future research with an animal model (Zimba et al. 2016). In 2017, scientists evaluated euglenophycin presence in 12 Euglena species and discovered concentrations > 5fg/cell (fg is a femtogram and 1 fg = 10-15g) in Euglena clavata, Euglena anabaena, Euglena stellata, Euglena socialis and Euglena sanguinea.
This value was chosen because produced the correct ion structure (based in the structure of Euglena sanguinea) and was produced in enough concentration to have a biological impact. It’s important to note that Euglena socialis and Euglena sanguinea accumulated a euglenophycin concentration 100 and 1000 fold higher respectively, than other Euglenaceae (Zimba et al. 2017).
Random Fact
A random fact about Euglena species is that they are potentially immortal, meaning they don’t age.
Scientist can prove this with experiments, in this case, cell-population growth rates were constant in cultures of Euglena gracilis without exhibit senescence (the cessation of cell division), and there was no change in DNA patterns during the exponential growth phase of these cultures (when DNA telomeres shorten during each cell division cycle).
The mechanisms of the algal defense against stress could be the key to understand the antiaging process (Goto & Beneragama 2010).
Common Questions
| Questions | Answers |
|---|---|
| Which Kingdom does euglena belong to? | Excavata |
| Is euglena prokaryotic or eukaryotic? | Euglena is eukaryotic. |
| Is euglena autotrophic or heterotrophic? | Euglena is classified as both heterotrophic and autotrophic because euglena can consume bacteria and other smaller microorganisms or use the energy produced by chloroplasts throughout the cell of the euglena. |
| Is euglena plant or animal? | Although Euglena can move, consume food, and obtain nutrients from photosynthetic chloroplasts through the cell, they are neither plant nor animals instead they fall in the Kingdom Excavata. |
| Do euglena have cell walls? | Unlike plants Euglena do not have a cell wall. Instead they have a flexible pellicle that allows them to perform their famous shape shifting. |
| Why are Euglena green? | Euglena are green because of the symbiotic chloroplasts found throughout the Euglena cell. Chloroplasts are green because of the presence of chlorophyll which helps plants absorb energy from the sun. |
| How are Euglena similar to plants? | They are similar to plants in that are able to absorb nutrients from chloroplasts which produce those nutrients during photosynthesis which is how plants produce food for themselves. |
| Euglena use which structure for movement? | Euglena primarily use a whip like structure called “flagella” for movement. |
| What happens to a euglena when sunlight is not present? | When sunlight is not present Euglena will begin to absorb organic nutrients heterotrophically. |
| Which part of euglena is responsible for respiration? | The organelle responsible for respiration in Euglena is mitochondria. |
Takeaways
To sum up, the Euglena genus is comprised of microscopic eukaryotic organisms with oval shape, chloroplasts (that synthesize paramylon) and two flagella that live in freshwater, saltwater or moist soils.
They are capable of creating their own energy by photosynthesis or feed on other microorganisms and have the ability to change the source of their energy according to light conditions. This genus has some incredible species, Euglena gracilis, the most studied, is used in the improvement of food nutritional value for human consumption and livestock.
It has the potential to serve as biofuel and decrease greenhouses emissions in the near future. Other species have a wide range of harsh conditions in which they can survive and live.
A particular species of Euglena produces a toxic alkaloid called euglenophycin capable of killing fish and other algae exposed to large concentrations of the toxin and at the same time have anticancer activity, in vitro. So much for “pond scum”!
References
- Aemiro, A., Watanabe, S., Suzuki, K., Hanada, M., Umetsu, K., & Nishida, T. (2016). Effects of Euglena (Euglena gracilis) supplemented to diet (forage: concentrate ratios of 60: 40) on the basic ruminal fermentation and methane emissions in in vitro condition. Animal Feed Science and Technology, 212, 129-135.
- Ashlock, P. D. (1971). Monophyly and associated terms. Systematic Biology, 20(1), 63-69.
- Dobell, C. (1923). A protozoological bicentenary: Antony van Leeuwenhoek (1632–1723) and Louis Joblot (1645–1723). Parasitology, 15(3), 308-319.
- Fryxell, G. A. (Ed.). (1983). Survival strategies of the algae. CUP Archive.
- Gissibl, A., Sun, A., Care, A., Nevalainen, H., & Sunna, A. (2019). Bioproducts from Euglena gracilis: Synthesis and applications. Frontiers in bioengineering and biotechnology, 7, 108.
- Goto, K., & Beneragama, C. K. (2010). Circadian clocks and antiaging: Do non-aging microalgae like Euglena reveal anything? Ageing research reviews, 9(2), 91-100.
- Karnkowska, A., Bennett, M. S., Watza, D., Kim, J. I., Zakryś, B., & Triemer, R. E. (2015). Phylogenetic relationships and morphological character evolution of photosynthetic euglenids (Excavata) inferred from taxon‐rich analyses of five genes. Journal of Eukaryotic Microbiology, 62(3), 362-373.
- Kim, J. I., Linton, E. W., & Shin, W. (2015). Taxon-rich multigene phylogeny of the photosynthetic euglenoids (Euglenophyceae). Frontiers in Ecology and Evolution, 3, 98.
- Montegut‐Felkner, A. E., & Triemer, R. E. (1997). Phylogenetic relationships of selected euglenoid genera based on morphological and molecular data. Journal of phycology, 33(3), 512-519.
- Radzikowski, J. (2013). Resistance of dormant stages of planktonic invertebrates to adverse environmental conditions. Journal of Plankton Research, 35(4), 707-723.
- Ruggiero, M. A., Gordon, D. P., Orrell, T. M., Bailly, N., Bourgoin, T., Brusca, R. C., Cavalier-Smith, T., Guiry, M. D. & Kirk, P. M. (2015). Correction: a higher level classification of all living organisms. Plos one, 10(6).
- Sánchez, E., Vargas, M., Mora, M., Ortega, J. M., Serrano, A., Freer, E., & Sittenfeld, A. (2004). Descripción ultraestructural de Euglena pailasensis (Euglenozoa) del Volcán Rincón de la Vieja, Guanacaste, Costa Rica. Revista de biología tropical, 52(1), 31-40.
- Singleton, W. (2018). Invertebrate Reproduction and Development. ED-Tech Press. 356 pp.
- Sittenfeld, A., M. Mora, J.M. Ortega, F. Albertazzi, A. Cordero, M. Roncel, E. Sánchez, M. Vargas, M. Fernández, J. Weckesser & A. Serrano. (2002). Characterization of a photosynthetic Euglena strain isolated from an acidic hot mud pool of a volcanic area of Costa Rica. FEMS Microbiol. Ecol. 42: 151-161.
- Watanabe, M., & Suzuki, T. (2004). Cadmium-induced synthesis of HSP70 and a role of glutathione in Euglena gracilis. Redox report, 9(6), 349-353.
- Zakryś, B., Milanowski, R., & Karnkowska, A. (2017). Evolutionary origin of Euglena. In Euglena: Biochemistry, cell and molecular biology (pp. 3-17). Springer, Cham.
- Zimba, P. V., Rowan, M., & Triemer, R. (2004). Identification of euglenoid algae that produce ichthyotoxin (s).
- Zimba, P. V., Ordner, P., & Gutierrez, D. B. (2016). Selective toxicity and angiogenic inhibition by euglenophycin: a role in cancer therapy. J Cancer Biol Treat, 3(008).
- Zimba, P. V., Moeller, P. D., Beauchesne, K., Lane, H. E., & Triemer, R. E. (2010). Identification of euglenophycin–A toxin found in certain euglenoids. Toxicon, 55(1), 100-104.
- Zimba, P. V., Huang, I. S., Gutierrez, D., Shin, W., Bennett, M. S., & Triemer, R. E. (2017). Euglenophycin is produced in at least six species of euglenoid algae and six of seven strains of Euglena sanguinea. Harmful algae, 63, 79-84.