The genus “Daphnia” is a group of aquatic organisms which are commonly known as “water fleas” and can range in body length from 0.2–8.0 mm with the exception of Leptodora, which measures up to 18 mm. Daphnia are commonly found in freshwater. They live in almost every nutrient-rich (eutrophic) body of water. Only, a few daphnia species are believed to be marine.
These tiny organisms are called water fleas simply because they swim in a jerky or hopping pattern. However, the name water flea is not specific to daphnia alone and it can be used for another organism that swim in a similar manner.
Daphnia is an interesting organism not only for being the primary key consumer of the food chain but also as a model organism for scientific studies. Daphina are of great interest to researchers, especially to ecologists and geneticists. Scientists study them for phenotypic plasticity which is the ability to achieve more than one phenotype from a single genotype. This feature is extremely beneficial when they are exposed to adverse environments. For them it is a kind of defense system against predators.
During the summer season, when daphnia are more prone to predator attacks, they generate enlarged spines and a “helmet” to protect themselves. The create the helmet by elongating and hardening their head with the help of special hormones. This change reduces the success of predators towards daphnia as a food. It is also metabolically advantageous because it saves them a lot on associated energy costs when the defense is unnecessary, in the absence of a predator population.
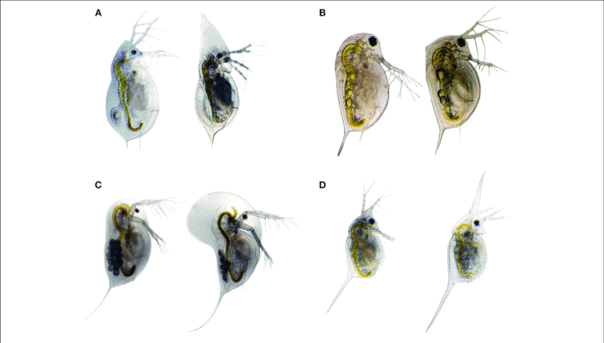
Another phenotypic plasticity example is their capability of changing the swimming behavior which is also known as “vertical migration”. They move vertically to swim at different water depths based on the change in temperature or daylight.
Daphnia are capable of reproductive switching between asexual and sexual reproduction. This is again a response to adverse environmental change. The scientists who are keen to learn about the evolution of sexual and asexual reproduction see this as a fascinating feature. Other than the large predators of daphnia such as fish, some bacterial, fungi, and algae species are also natural enemies of daphnia. These behave like a parasite or an epibiont that either live inside or on to the daphnia body. This provides another huge opportunity for the study of host-parasite relationships and toxicological studies.
Classification of Daphnia

Daphnia is a planktonic crustacean. Planktonic organisms stay afloat in large water bodies but are incapable of swimming against the water current. They are the vital food source for fish and many other aquatic animals. The crustaceans, including crabs, lobsters, copepods, barnacles, and shrimps are a group of aquatic arthropods that have their limbs and antennae divided to form two branches (biramous).
Daphnia belongs to phylum Phyllopoda, also known as Branchiopoda. Their main characteristic anatomical feature is flattened leaf-like legs which helps them producing the local water current, like a boat raft. These also play an interesting role in the feeding mechanism. Within the branchiopods, genusDaphnia belongs to order Cladocera, since their bodies are enclosed by an uncalcified shell, technically named as the carapace. It is kind of an exoskeleton which is mostly made up of fibrous polysaccharide, chitin.
The genus Daphnia includes more than 100 known species around the world. They usually inhabit most types of standing fresh water except for a few extreme habitats, such as hot springs. Most of the species are filter feeders and float freely. However, at times they can also be found clinging to water plants or the bottom sediments of shallow ponds.
The two most common daphnia species are D. pulex and D. magna. These are often associated with another organism “Moina” of a related genus of Cladocera. Moina belongs to the Moinidae family instead of the Daphniidae family of daphnia. It is also much smaller in size, almost half, compared to the length of D. pulex. Among them, as the name suggests, D. magna is the largest, however D. pulex is the one that is more commonly found. The other not so common daphnia species are D. galeata, D. cucullata, and D. hyalina.
Daphnia Anatomy
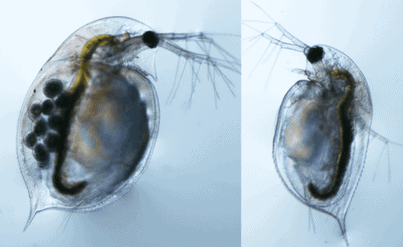
Daphnia main body is secured by a chitin shell carapace. The shell is often clear which makes it easier to study the animal under a microscope. Even with relatively low power magnifications, processes like feeding, eye movement, heart and circulatory system, and the movement of immature young within the female bodies can be easily observed. It is amazing that daphnia is tolerant and suffers no harm under a coverslip, while observing under a microscope. They can be happily returned to their habitat after observation!
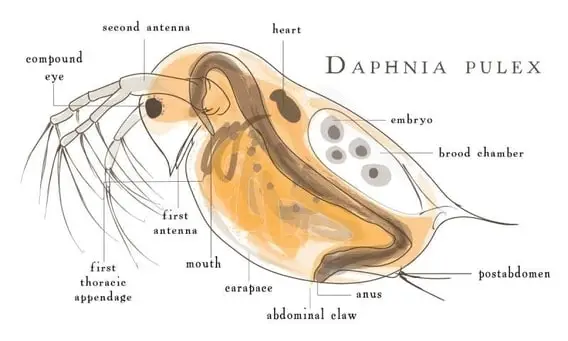
When the young daphnia grow larger, the body shell cannot grow. Hence, daphnia have evolved to molt and get rid of the old shell. The new shell usually starts growing under the old one. This makes sure that the organism remains shielded at all times. When the old shell is discarded, daphnia quickly absorbs the water into the new one. This rapid stage of producing a shell is called “Instar”. The young daphnia that is released from the mother body into the water needs to pass through 4–6 instars before they turn mature enough to reproduce. Interestingly, the juveniles can almost double their size from one instar to the next.
The daphnia body is segmented. It has a fused head that contains a dark-colored compound eye. The cerebral ganglion forms the major part of the nervous system and is also located near the eye. In the beginning, the embryo has two brownish eye spots which in later developmental stages, get fused as one large compound eye in the adult animal. The eye helps the animal to position while swimming.
The other distinguishing features of daphnia are a second set of antennae and a pair of abdominal hairs called setae. Daphnia has up to 10 pairs of appendages, which are antennules, antennae maxillae, and mandibles, followed by 5 limbs on the trunk. However, in other Cladocera organisms, there are 6 limbs on the trunk. The limbs function as a filtering apparatus that helps in feeding and also assist respiration.
There is also a pair of claws at the end of the abdomen. Though the males are smaller in size, they got larger antennules. These are armed with a hook-like structure to tightly grip the female during sexual activity. Comparatively, the body of female daphnia are much bigger, simply because of the brood chamber that holds several eggs.
Daphnia gut is tubular and can be anatomically divided into the esophagus, the midgut, and the hindgut. There are also two small digestive ceca. These ceca functions similar to the intestine in humans. Food is passed by peristaltic contractions of the gut. The midgut is lined with an epithelium which absorbs molecules and do not phagocytose particles like other microorganisms. The average pH of the anterior gut is 6.2 and transitions to 7.2 in the later part. The digested food gets expelled from the hindgut from the pressure of the recently engulfed food. This pressure is required in addition to the peristaltic movement of the gut.
Starved animals are transparent, however, they turn greenish-yellow or pinkish based on feeding on green algae or bacteria.
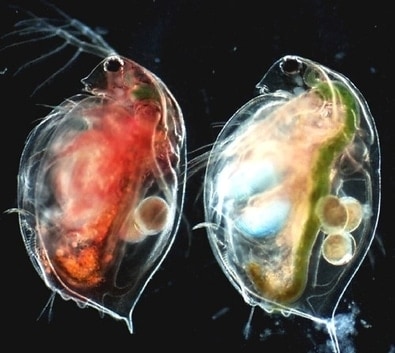
Daphnia has an open blood circulation system. Theheart is located dorsally anterior from the brood chamber. The heart beats about 200 times per minute, at 20ºC, and is sensitive to the environmental temperature. The heartbeat will get slower in cold water.
The purpose of blood flow in daphnia is similar to humans in that oxygen is transported by protein hemoglobin throughout the body. Interestingly, when the oxygen level gets lower, daphnia can increase hemoglobin production twenty fold. This increases the oxygen uptake from the water and helps to fight hypoxic conditions.
Daphnia Lifecycle and Reproduction
The lifespan of daphnia is highly dependent on the temperature and adversity of the environment. At 3°C, they live up to 108 days, and at 28°C for only 29 days. However, during the wintertime in harsh conditions some females can live longer than six months.
These females grow at a much slower rate but in the end, are much larger than normal ones. In favorable conditions, from spring till summer, daphnia reproduces asexually (parthenogenetically). The fully mature females produce young about every eight to ten days. Juveniles are nurtured in the brood pouch of the mother. However, after hatching, the young daphnia need to molt several times to turn into an adult. This process usually takes about two weeks. The type of asexual reproduction process continues until environmental conditions turn unfavorable.
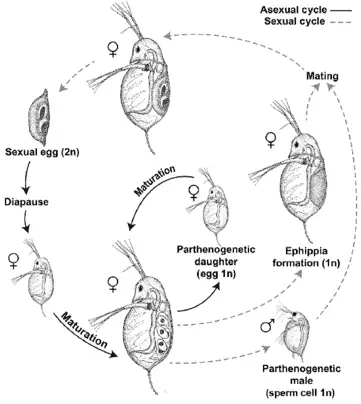
In the harsh season of winter or drought conditions, some males are also produced. It is interesting that both females and males can be produced parthenogenically. The males fertilize the eggs which are termed winter eggs (ephippia). These eggs have a protective shell that protects them from the harsh environmental conditions until a more favorable season arrives.
Then, once again, the normal asexual reproductive cycle takes over. This switching from asexual to sexual reproduction offers greater genetic variation in offspring. This is of great interest to scientists who intend to understand the mechanism of such switching and the overall process of evolution by genetic variation in daphnia.
Daphnia Habitat
In nature, the distributionof specific daphnia species is highly dependent on the presence of its predators. Usually, the smaller species are prominent in lakes with planktivorous fish whereas more transparent species are found in fishless water bodies. It is relatively difficult for the large species such as D. magna and D. pulex to survive with the predators.
Though the water quality of the habitats can vary widely from pH 6.5 to 9.5, an optimum pH is around 7.8. Daphnia also like the optimal salinity below 5% of seawater. However, some species like D. magna can tolerate up to 20% seawater. Daphnia can actually be found in a variety of aquatic environments, ranging from acidic swamps to freshwater lakes, ponds, streams, and rivers.
Though daphnia is mostly a filter feeder, at times it can also prey on other tiny crustaceans and rotifers. It can also feed on unicellular algae, organic debris, protists, and bacteria. The beating of legs produces a current of water flow through the carapace that pushes the food particles towards the gut. The second set of antennae is larger and influences swimming in the characteristic “water flea” jumping motion.
However, daphnia cannot sustain strong water current, due to its inability to swim against it. Usually, being light enough, they just remain suspended and float with the water current in the algae-rich upper portion of the water column. Their leaf-like structure is especially helpful to achieve this. But, depending on the type of predators and/or climate, they migrate vertically in the water bed. This changes their relative position along the water depth. Though this is a high energy process, it helps to avoid the predators towards the surface at night.
The phytoplankton food supply is also controlled by seasonal variation, so that is another reason for the vertical movement of daphnia. Interestingly, in fish tanks, daphnia can be used to clear algae bloom. However, blue-green algae is a harmful food for daphnia, due to the tough outer cell wall. Daphnia is also a popular live food that is used for tropical and marine fish keeping and small amphibians such as tadpoles.
The nutritional value of daphnia depends on the chemical composition of their food source. However, being a freshwater species, daphnia are not a suitable food for marine organisms. Daphnia can be commercially cultivated in tanks or large ponds. The inoculation is carried out using the resting eggs or adult animals. Daphnia population grows fast as it takes only eight to ten days for a juvenile to mature and start breeding. A population of 100 daphnia can reach up to 1000 in a week and to 10,000 by another week.
In a tank, they can live on active dry yeast. One can also use spirulina powder as an algae superfood. Daphnia can also be cultured in large ponds of water beds with 5 feet in height. The resting eggs (ephippia) are the inoculating material for the ease of storage and shipment. Their production can be induced by exposing the daphnia population to a combination of stress, such as low food, lower temperatures, animal crowding, and/or short daylight periods.
This is comparatively easier with an aging population. Collection of the ephippia is done by taking sediment samples and passing them through a 200 µm sieve. Later, ephippia are isolated under a binocular microscope form this sample.
Daphnia and Scientific Inquiry
Daphnia is a broadly accepted model system to study ecology, genetics, epigenetics, and host-parasite toxicology. The small reproductive cycle of only 8-10 days makes it ideal for the experimental genetics. The distinct lineages of daphnia that were independently colonized in radically different environments are available in various scientific labs. Daphnia are also good for comparative genomic studies among arthropods. The crustaceans and insects diverged from a common ancestor and therefore genomic characteristics are preserved in the daphnia genome.
In evolutionary biology, environmental adaption is a fundamentally important phenomenon. It is key to understand the mechanism that brings the underlying changes in animal morphology, physiology, and behavior. These are often related to the modifications in the corresponding genes. D. pulex, with24 chromosomes is the first species to be sequenced among the crustaceans.
Most other daphnia species have only 20 chromosomes. The growing number of research publications on daphnia further emphasizes its importance. It is probably the best-studied animal subject in ecology. The ecology studies are focused primarily on phenotypic plasticity, behavior, toxicology, and the evolution of sexual and asexual reproduction. The population genetics studies try to understand migration and gene flow, hybridization, and inbreeding in daphnia.
Several of the mentioned features of daphnia are induced by environmental cues. The change in morphology (development of spine and helmet) and behavior (vertical movement) are induced by predator-borne chemical cues. These are known as kairomones. In the presence of fish kairomones, D. magna starts producing smaller offspring, whereas chemical cues from the phantom midge, Chaoborus flavicans induce the generation of larger progeny.
This is an advantageous adaptive phenotypic plasticity to avoid predation as fish and midges prefer different sizes of prey. Other than these predators, viruses, bacteria, and fungi are also natural enemies of daphnia. These have a larger influence on daphnia ecology and evolution. The hosts continuously evolve to fight the parasite whereas parasites evolve to keep virulence intact. Scientists found that certain molecular signaling pathway are activated in the presence of pathogens. These are evolutionarily conserved between insects and daphnia. It is suggested that the activation of the pathway produces antibacterial and antifungal proteins.
The unusual life cycle of the daphnia has been under investigation for more than a century for cyclic parthenogenesis. It can produce both diploid (asexual) and haploid sexual eggs. As discussed previously in this post sex determination is controlled by environmental parameters. Males are produced in response to harsh conditions so that better protected sustainable eggs (ephippia) can be produced.
Daphniaalsoshow spectacular polyphenisms. This is the capability to produce alternative phenotypes such as the formation of the helmet, neck teeth, and spine. This makes it an excellent model to study the epigenetic developmental programs. Epigenetic changes are heritable changes in phenotypes that occur without altering the DNA sequence. These are caused by DNA methylation, histone modifications, and RNA interference, and other less understood highly complex mechanisms.
As an epigenetic model, daphnia offers a variety of benefits since it allows the study of epigenetic effects in the absence of complex genetic differences. It is important to note that the young are usually produced asexually and hence the offspring is a genetic clone of the mother. Apart from morphological changes, even the sex determination and sexual reproduction are epigenetically determined.
In the case of daphnia, it is found that DNA methylation has been altered in response to toxicants and heavy metals, although the investigation of other epigenetic mechanisms has only started in recent years. More investigation for histone modifications and RNAi (RNA interference) in sex determination and predator-induced defenses are underway to expand our scientific understanding.
The diets of various species not only satisfy everyday metabolic requirements of organisms but can also serve as “medicines” to combat disease and infection. A laboratory-based study shows that daphnia can acquire protection from fungal parasites by consuming toxins produced by bloom-forming cyanobacteria. Daphnia synthesize secondary metabolites that might have antimicrobial properties.
Daphnia are being investigated for such medicinal properties using a toxin-producing diet against fungal parasites. In this particular study, daphnia living in Michigan lakes were fed green algae and toxic cyanobacteria diets. They were then exposed to fungal and bacterial parasites. The experimental results showed that daphnia gained protection from fungal parasites through the toxins that were present in bloom-forming cyanobacteria. This opens up potential research avenues that could result in novel anti-fungal drugs for human use.
Takeaways
In a nutshell, daphnia is an intriguing tiny transparent freshwater organism. It is at the bottom of the ecological pyramid (food chain pyramid) and serves as a planktonic food for fishes and many other small organisms. Daphnia feed on algae, fungi, and bacteria.
Anatomically, daphnia is a simple organism that mostly reproduces asexually. Its unique capabilities to adapt against harsh weather and predators make it an excellent animal model to answer significant questions of modern biology. The genome of daphnia was recently sequenced and that will significantly speed up our learning even further.
If you ever run across one of these transparent “water fleas” while observing a pond sample, you will be in for a treat!
References
- Miller C. Daphnia pulex [Internet]. Animal Diversity Web. [cited 2020 May 5]. Available from: https://animaldiversity.org/accounts/Daphnia_pulex/
- Water flea | crustacean [Internet]. Encyclopedia Britannica. [cited 2020 May 6]. Available from: https://www.britannica.com/animal/water-flea
- Weiss LC, Leimann J, Tollrian R. Predator-induced defences in Daphnia longicephala: location of kairomone receptors and timeline of sensitive phases to trait formation. J Exp Biol. 2015 Sep 1;218(18):2918–26. https://jeb.biologists.org/content/218/18/2918
- Haupt F, Stockenreiter M, Baumgartner M, Boersma M, Stibor H. Daphnia diel vertical migration: implications beyond zooplankton. J Plankton Res. 2009 May 1;31(5):515–24. https://doi.org/10.1093/plankt/fbp003
- Zhang Y-N, Zhu X-Y, Wang W-P, Wang Y, Wang L, Xu X-X, et al. Reproductive switching analysis of Daphnia similoides between sexual female and parthenogenetic female by transcriptome comparison. Sci Rep. 2016 Sep 27;6(1):1–9. https://doi.org/10.1038/srep34241
- Ebert D, Ebert D. Ecology, Epidemiology, and Evolution of Parasitism in Daphnia. National Center for Biotechnology Information (US); 2005. https://www.ncbi.nlm.nih.gov/books/NBK2036/
- Daphnia. In: Wikipedia [Internet]. 2020 [cited 2020 May 5]. Available from: https://en.wikipedia.org/w/index.php?title=Daphnia&oldid=953932159
- Oneida Lake Education Initiative [Internet]. [cited 2020 May 5]. Available from: https://seagrant.sunysb.edu/OLI/olei-daphnia.htm
- Daphnia – New World Encyclopedia [Internet]. [cited 2020 May 5]. Available from: https://www.newworldencyclopedia.org/entry/Daphnia
- Daphnia and Moina [Internet]. [cited 2020 May 7]. Available from: http://www.fao.org/3/w3732e/w3732e0x.htm#6.1.%20Daphnia%20and%20Moina
- Co-Op A. Daphnia Culturing – How to Raise Daphnia [Internet]. Aquarium Co-Op. [cited 2020 May 7]. Available from: https://www.aquariumcoop.com/blogs/aquarium/daphnia-culturing-how-to-raise-daphnia
- Stollewerk A. The water flea Daphnia – a “new” model system for ecology and evolution? J Biol. 2010;9(2):21. https://doi.org/10.1186/jbiol212
- Harris KDM, Bartlett NJ, Lloyd VK. Daphnia as an Emerging Epigenetic Model Organism [Internet]. Vol. 2012, Genetics Research International. Hindawi; 2012 [cited 2020 May 6]. p. e147892. Available from: https://www.hindawi.com/journals/gri/2012/147892/
- Proceedings of the Royal Society B: Biological Sciences [Internet]. [cited 2020 May 8]. Available from: https://royalsocietypublishing.org/doi/10.1098/rspb.2018.2231

